Difference between revisions of "Diphenylcarbazide"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | White crystalline powder used for the colorimetric detection of [ | + | White crystalline powder used for the colorimetric detection of [[chromium]] in metals, tanned [[leather]], and [[pigment|pigments]] (Odegaard et al 2000). Diphenylcarbazide gives a dark blue-violet positive reaction. Other metals, such as [[cadmium]], [[mercury]], [[magnesium]], [[silver]], [[nickel]], [[tin]], and [[aluminum]] will also react with diphenylcarbazide. |
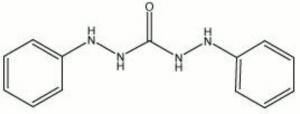
| − | + | [[[SliderGallery rightalign|diphenylcarbazide.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
2,2'-diphenylcarbonic dihydrazide; 1,5-diphenylcarbazide; S-diphenylcarbazide; DPC | 2,2'-diphenylcarbonic dihydrazide; 1,5-diphenylcarbazide; S-diphenylcarbazide; DPC | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Degrades in light. | ||
| + | * May be harmful by ingestion or inhalation. | ||
| + | * Contact may cause irritation. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC158840250&productDescription=SYM.-DIPHENYLCARBAZIDE+25GR&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Slightly soluble in water. Soluble in hot alcohol, acetone, glacial acetic acid | Slightly soluble in water. Soluble in hot alcohol, acetone, glacial acetic acid | ||
| Line 22: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 168-171 | + | | 168-171 C |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 28: | Line 33: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 3333 | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 3333 | ||
| − | * N.Odegaard, S.Carroll, W.Zimmt, ''Material Characterization Tests for Objects of Art and Archaeology'', Archetype Publications, London, 2000 | + | * N.Odegaard, S.Carroll, W.Zimmt, ''Material Characterization Tests for Objects of Art and Archaeology'', Archetype Publications, London, 2000, p.44. |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 16:04, 21 July 2022
Description
White crystalline powder used for the colorimetric detection of Chromium in metals, tanned Leather, and pigments (Odegaard et al 2000). Diphenylcarbazide gives a dark blue-violet positive reaction. Other metals, such as Cadmium, Mercury, Magnesium, Silver, Nickel, Tin, and Aluminum will also react with diphenylcarbazide.
Synonyms and Related Terms
2,2'-diphenylcarbonic dihydrazide; 1,5-diphenylcarbazide; S-diphenylcarbazide; DPC
Risks
- Degrades in light.
- May be harmful by ingestion or inhalation.
- Contact may cause irritation.
- ThermoFisher: SDS
Physical and Chemical Properties
Slightly soluble in water. Soluble in hot alcohol, acetone, glacial acetic acid
| Composition | C33H14N4O |
|---|---|
| CAS | 140-22-7 |
| Melting Point | 168-171 C |
| Molecular Weight | mol. wt. = 242.27 |
Resources and Citations
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 3333
- N.Odegaard, S.Carroll, W.Zimmt, Material Characterization Tests for Objects of Art and Archaeology, Archetype Publications, London, 2000, p.44.