Difference between revisions of "Calcium carbonate"
| (6 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
A white powder that can occur in three crystalline forms: [[calcite]] (hexagonal-rhombohedral), [[aragonite]] (orthorhombic) and vaterite. Calcium carbonate occurs naturally in many forms such as [[chalk]], [[limestone]], [[marble]] and [[seashell|sea shells]]. It can be found worldwide and ranges in color (because of impurities) from white to gray to yellow. A white pigment of calcium carbonate is prepared by grinding limestone, chalk or shells with water then using levigation to separate the coarser material. Artificial chalk, also known as precipitated chalk, is whiter and more homogeneous than natural chalk. Pearl white is made from calcined oyster shells. | A white powder that can occur in three crystalline forms: [[calcite]] (hexagonal-rhombohedral), [[aragonite]] (orthorhombic) and vaterite. Calcium carbonate occurs naturally in many forms such as [[chalk]], [[limestone]], [[marble]] and [[seashell|sea shells]]. It can be found worldwide and ranges in color (because of impurities) from white to gray to yellow. A white pigment of calcium carbonate is prepared by grinding limestone, chalk or shells with water then using levigation to separate the coarser material. Artificial chalk, also known as precipitated chalk, is whiter and more homogeneous than natural chalk. Pearl white is made from calcined oyster shells. | ||
| − | + | [[File:mcalcite plmp.jpg|thumb|Plane polarized light micrograph of calcite]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
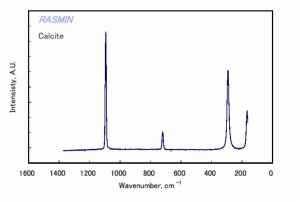
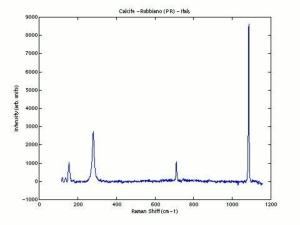
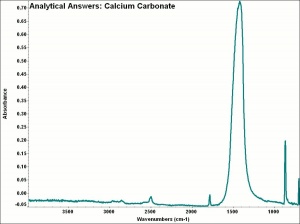
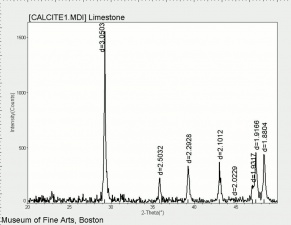
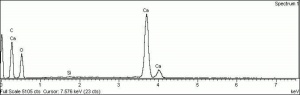
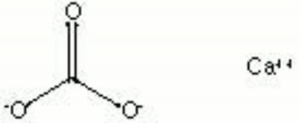
[[[SliderGallery rightalign|calciteRS.jpg~Raman|Calciteitaly1.jpg~Raman|aaiCACO3.jpg~FTIR|CALCITE1.jpg~XRD|fwhitingsem.jpg~SEM|fwhitingedsbw.jpg~EDS|calcium carbonate.jpg~Chemical structure]]] | [[[SliderGallery rightalign|calciteRS.jpg~Raman|Calciteitaly1.jpg~Raman|aaiCACO3.jpg~FTIR|CALCITE1.jpg~XRD|fwhitingsem.jpg~SEM|fwhitingedsbw.jpg~EDS|calcium carbonate.jpg~Chemical structure]]] | ||
| + | == Risks == | ||
| + | |||
| + | No significant hazards. | ||
| − | == | + | ThermoFisher: [https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-c/S25220.pdf SDS] |
| + | == Physical and Chemical Properties == | ||
| − | Particle size = 0.1-10 micrometers. | + | * Particle size = 0.1-10 micrometers. |
| + | * Insoluble in water. | ||
| + | * May fluoresce a medium purple color in ultraviolet light. | ||
| + | * Reacts with acids to evolve carbon dioxide. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 23: | Line 30: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.7-2.95 | + | | 2.7-2.95 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 38: | ||
| 1.510; 1.645 | | 1.510; 1.645 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 48: | Line 45: | ||
[[media:download_file_512.pdf|Characteristics of Common White Pigments]] | [[media:download_file_512.pdf|Characteristics of Common White Pigments]] | ||
| − | + | ==Resources and Citations== | |
| − | + | * R. Gettens, E. West Fitzhugh, R.Feller, "Calcium Carbonate Whites", ''Artists Pigments'', Vol. 2., A. Roy ed. Oxford University Press, Oxford, 1993. | |
| − | == | ||
| − | |||
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive index: 1.64-1.66; 1.486 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive index: 1.64-1.66; 1.486 | ||
| Line 76: | Line 71: | ||
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | * Website | + | * Website: www.hants.org.uk/museums - conservation termlist |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 08:08, 18 May 2022
Description
A white powder that can occur in three crystalline forms: Calcite (hexagonal-rhombohedral), Aragonite (orthorhombic) and vaterite. Calcium carbonate occurs naturally in many forms such as Chalk, Limestone, Marble and sea shells. It can be found worldwide and ranges in color (because of impurities) from white to gray to yellow. A white pigment of calcium carbonate is prepared by grinding limestone, chalk or shells with water then using levigation to separate the coarser material. Artificial chalk, also known as precipitated chalk, is whiter and more homogeneous than natural chalk. Pearl white is made from calcined oyster shells.
Synonyms and Related Terms
chalk; carbonate de calcium (Fr.); Kalziumkarbonat (Deut.); carbonato cálcico (Esp.); carbonato de calcio (Esp.); calciumcarbonaat (Ned.); gofun (Jap.); aragonite; pearl white; oystershell white; shell white; marble; limestone, whiting; lime white; marl; travertine; Pigment White 18; white earth; English white; Paris white; drop chalk
Risks
No significant hazards.
ThermoFisher: SDS
Physical and Chemical Properties
- Particle size = 0.1-10 micrometers.
- Insoluble in water.
- May fluoresce a medium purple color in ultraviolet light.
- Reacts with acids to evolve carbon dioxide.
| Composition | CaCO3 |
|---|---|
| CAS | 471-34-1 |
| Density | 2.7-2.95 g/ml |
| Molecular Weight | mol. wt. = 100.09 |
| Refractive Index | 1.510; 1.645 |
Comparisons
Properties of Common Abrasives
Characteristics of Common White Pigments
Resources and Citations
- R. Gettens, E. West Fitzhugh, R.Feller, "Calcium Carbonate Whites", Artists Pigments, Vol. 2., A. Roy ed. Oxford University Press, Oxford, 1993.
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive index: 1.64-1.66; 1.486
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 2.70 and ref. index = 1.510; 1.645
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: ref. index = 1.66; 1.44
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Website: www.hants.org.uk/museums - conservation termlist