Difference between revisions of "Anhydrite"
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:65.1749-SC30364.jpg|thumb|]] | + | [[File:65.1749-SC30364.jpg|thumb|Egyptian ointment jar<br>MFA# 65.1749]] |
== Description == | == Description == | ||
| Line 10: | Line 10: | ||
anhydrous calcium sulfate; Anhydrit (Deut.); anhydrite (Fr.); anhidrita (Esp.); anydritis (Gr.); anidrite (It.); anhydriet (Ned.); anhydryt (Pol.); anidrite (Port.); blue marble; anhydrite white; | anhydrous calcium sulfate; Anhydrit (Deut.); anhydrite (Fr.); anhidrita (Esp.); anydritis (Gr.); anidrite (It.); anhydriet (Ned.); anhydryt (Pol.); anidrite (Port.); blue marble; anhydrite white; | ||
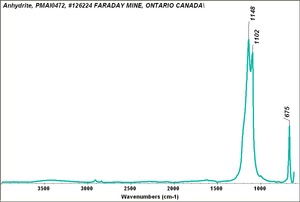
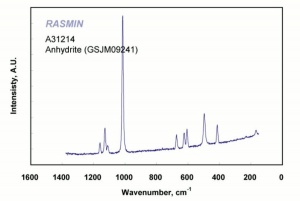
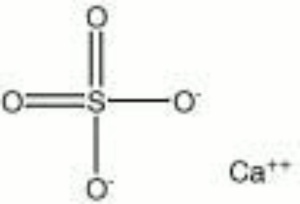
| − | [[[SliderGallery rightalign|Anhydrite PMA.TIF~FTIR (PMA)|anhydriteRS.jpg~Raman|anhydrite.jpg~Chemical structure]]] | + | [[[SliderGallery rightalign|Anhydrite PMA.TIF~FTIR (PMA)|anhydriteRS.jpg~Raman (RASMIN)|anhydrite.jpg~Chemical structure]]] |
| − | == | + | == Risks == |
| − | + | * Can alter to gypsum in humid conditions. | |
| + | * USG: [https://www.usg.com/content/dam/USG_Marketing_Communications/united_states/sds/usg-anhydrite-rock-sds-en-52000000153.pdf SDS] | ||
| − | + | == Physical and Chemical Properties == | |
| − | Under cross | + | * Orthorhombic crystals but usually massive. |
| + | * Perfect cleavage in three directions. | ||
| + | * Sometimes fluorescent, especially after heating. | ||
| + | * Fracture = uneven. | ||
| + | * Luster = vitreous to pearly. | ||
| + | * Streak = white to gray. | ||
| + | * Under cross polars, particles usually show bright second order interference | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 32: | Line 39: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 1450 | + | | 1450 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.9-3.0 | + | | 2.9-3.0 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| − | | | + | | 136.14 |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| 1.570; 1.575; 1.614 | | 1.570; 1.575; 1.614 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
| Line 64: | Line 59: | ||
</gallery> | </gallery> | ||
| + | ==Resources and Citations== | ||
| − | + | * B.Fay ""Egyptian Duck Flasks of Blue Anhydrite" in ''Metropolitan Museum Journal'', 33:23-48, 1998. | |
| − | + | * Mineralogy Database: [http://www.webmineral.com/data/Anhydrite.shtml Anhydrite] | |
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive Index: alpha=1.569-1.574; beta=1.574-1.579; gamma=1.609-1.618 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive Index: alpha=1.569-1.574; beta=1.574-1.579; gamma=1.609-1.618 | ||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 2.93; refractive index = 1.570; 1.614; 1.575 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 2.93; refractive index = 1.570; 1.614; 1.575 | ||
| − | |||
* ''Encyclopedia Britannica'', http://www.britannica.com Comment: "anhydrite" [Accessed December 4, 2001]. | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "anhydrite" [Accessed December 4, 2001]. | ||
| − | |||
* C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | ||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 38 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 38 | ||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 | ||
| − | + | * Wikipedia: [https://en.wikipedia.org/wiki/Anhydrite Anhydrite] (Accessed December 4, 2001 and July 2023). | |
| − | * Wikipedia | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 Comment: transparent white pigment |
| − | |||
| − | * Art and Architecture Thesaurus Online, | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 13:00, 14 July 2023
Description
A naturally occurring mineral of anhydrous calcium sulfate that is often found in gypsum deposits. Anhydrite was first identified as a mineral in deposits at Innsbruck, Austria in 1794. Other major deposits occur in Canada (Ontario), and the U.S. (Arizona, New Mexico). Anhydrite can be transparent or translucent and has a lustrous sheen. It occurs in several colors such as white, gray, blue, pink, red, and lavender. Anhydrite was used in ancient Egypt for carved objects and vessels, many of which are in the shape of animals (Fay, 1998). Powdered anhydrite has also been found as a filler in the gesso grosso ground layers of Italian paintings and polychromed sculpture. Currently, the colorless, inert pigment is often used as a filler in paper, paints, and plastics.
Synonyms and Related Terms
anhydrous calcium sulfate; Anhydrit (Deut.); anhydrite (Fr.); anhidrita (Esp.); anydritis (Gr.); anidrite (It.); anhydriet (Ned.); anhydryt (Pol.); anidrite (Port.); blue marble; anhydrite white;
Risks
- Can alter to gypsum in humid conditions.
- USG: SDS
Physical and Chemical Properties
- Orthorhombic crystals but usually massive.
- Perfect cleavage in three directions.
- Sometimes fluorescent, especially after heating.
- Fracture = uneven.
- Luster = vitreous to pearly.
- Streak = white to gray.
- Under cross polars, particles usually show bright second order interference
| Composition | CaSO4 |
|---|---|
| CAS | 7778-18-9 |
| Mohs Hardness | 3.0 - 3.5 |
| Melting Point | 1450 C |
| Density | 2.9-3.0 g/ml |
| Molecular Weight | 136.14 |
| Refractive Index | 1.570; 1.575; 1.614 |
Additional Images
Resources and Citations
- B.Fay ""Egyptian Duck Flasks of Blue Anhydrite" in Metropolitan Museum Journal, 33:23-48, 1998.
- Mineralogy Database: Anhydrite
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive Index: alpha=1.569-1.574; beta=1.574-1.579; gamma=1.609-1.618
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 2.93; refractive index = 1.570; 1.614; 1.575
- Encyclopedia Britannica, http://www.britannica.com Comment: "anhydrite" [Accessed December 4, 2001].
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 38
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- Wikipedia: Anhydrite (Accessed December 4, 2001 and July 2023).
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 Comment: transparent white pigment