Difference between revisions of "Beeswax"
(username removed) |
|||
| (8 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:51.360-SC120628.jpg|thumb|]] | + | [[File:51.360-SC120628.jpg|thumb|Women's headdress<br<MFA 51.360]] |
== Description == | == Description == | ||
| + | [[File:Beeswax.jpg|thumb|Bees on wax honeycomb]] | ||
| + | A wax produced by many species of bees; the most common is ''Apis mellifica''. Beeswax is secreted by the worker bees to form honeycomb cells. The wax is prepared by melting the combs in hot water then filtering out the impurities which may contain resins, sugars, and plant materials. The age, diet, location, and species of bee affects the color and texture of the wax. Waxes can be soft or brittle with colors ranging from light yellow to dark brown. The darker varieties are often bleached by exposure to light and air or with chemicals. Beeswax contains about 10-14% hydrocarbons in addition to alcohols, fatty acids and esters. The primary component is myricyl palmitate (C15H31COOC30H61). [[Punic wax]] is refined beeswax. Beeswax has been used as a protective coating, candle, adhesive, paint binder, and plasticizer. | ||
| − | + | [[File:beeswax_overall.jpg|thumb|Beeswax block]] | |
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
bees wax; bees-wax; bee's wax; punic wax; crude beeswax; bleached beeswax; yellow beeswax; white beeswax; virgin beeswax; yellow wax; ghedda wax; cera colla; cera flava (Lat.); Bienenwachs (Deut.); cire d'abeille (Fr.); bijenwas (Ned.); | bees wax; bees-wax; bee's wax; punic wax; crude beeswax; bleached beeswax; yellow beeswax; white beeswax; virgin beeswax; yellow wax; ghedda wax; cera colla; cera flava (Lat.); Bienenwachs (Deut.); cire d'abeille (Fr.); bijenwas (Ned.); | ||
| − | |||
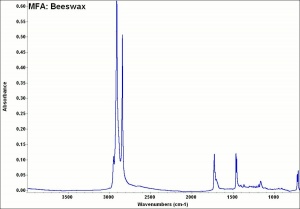
[[[SliderGallery rightalign|MFA- Beeswax.jpg~FTIR]]] | [[[SliderGallery rightalign|MFA- Beeswax.jpg~FTIR]]] | ||
| + | == Risks == | ||
| − | + | * Combustible. | |
| − | + | * Discolors when heated above 85 C. | |
| − | |||
| − | + | == Physical and Chemical Properties == | |
| − | Acid number = 16-24 | + | * Soluble in chloroform, ether, turpentine, oils. |
| + | * Forms an emulsion with alkalis. | ||
| + | * Slightly soluble in ethanol. Insoluble in water. | ||
| + | * For yellow beeswax: Saponification number = 84-97; Iodine number =6-11; Acid number = 16-24; Total % alcohols and hydrocarbons = 52-56 | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 62-70 | + | | 62-70 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.95-0.96 | + | | 0.95-0.96 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| 1.440-1.449 | | 1.440-1.449 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| − | [[media: | + | [[media:download_file_10.pdf|Properties of Natural Waxes]] |
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
<gallery> | <gallery> | ||
| − | + | File:beeswax_det.jpg|Beeswax, closeup | |
| − | File:beeswax_det.jpg|Beeswax | ||
File:60-78_Beeswax_canvas.jpg|Beeswax | File:60-78_Beeswax_canvas.jpg|Beeswax | ||
</gallery> | </gallery> | ||
| − | + | == Resources and Citations == | |
| − | == | ||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 78: | Line 69: | ||
* Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Beeswax (Accessed Oct. 18, 2005) |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Beeswax." | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Beeswax." Accessed 14 Apr. 2004. |
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
Latest revision as of 13:37, 2 May 2022
Description
A wax produced by many species of bees; the most common is Apis mellifica. Beeswax is secreted by the worker bees to form honeycomb cells. The wax is prepared by melting the combs in hot water then filtering out the impurities which may contain resins, sugars, and plant materials. The age, diet, location, and species of bee affects the color and texture of the wax. Waxes can be soft or brittle with colors ranging from light yellow to dark brown. The darker varieties are often bleached by exposure to light and air or with chemicals. Beeswax contains about 10-14% hydrocarbons in addition to alcohols, fatty acids and esters. The primary component is myricyl palmitate (C15H31COOC30H61). Punic wax is refined beeswax. Beeswax has been used as a protective coating, candle, adhesive, paint binder, and plasticizer.
Synonyms and Related Terms
bees wax; bees-wax; bee's wax; punic wax; crude beeswax; bleached beeswax; yellow beeswax; white beeswax; virgin beeswax; yellow wax; ghedda wax; cera colla; cera flava (Lat.); Bienenwachs (Deut.); cire d'abeille (Fr.); bijenwas (Ned.);
Risks
- Combustible.
- Discolors when heated above 85 C.
Physical and Chemical Properties
- Soluble in chloroform, ether, turpentine, oils.
- Forms an emulsion with alkalis.
- Slightly soluble in ethanol. Insoluble in water.
- For yellow beeswax: Saponification number = 84-97; Iodine number =6-11; Acid number = 16-24; Total % alcohols and hydrocarbons = 52-56
| Melting Point | 62-70 C |
|---|---|
| Density | 0.95-0.96 g/ml |
| Refractive Index | 1.440-1.449 |
Comparisons
Additional Images
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry # 1049
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 95
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- M. Doerner, The Materials of the Artist, Harcourt, Brace & Co., 1934
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Kurt Wehlte, The Materials and Techniques of Painting, Van Nostrand Reinhold Co., New York, 1975
- John S. Mills, Raymond White, The Organic Chemistry of Museum Objects, Butterworth Heineman, London, 2nd ed., 1994
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Wikipedia: http://en.wikipedia.org/wiki/Beeswax (Accessed Oct. 18, 2005)
- Encyclopedia Britannica, http://www.britannica.com Comment: "Beeswax." Accessed 14 Apr. 2004.
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: For yellow beeswax = melting point=62-65C, density=0.960-0.964, ref. index=1.443-1.449, iodine value=6-11, acid value=18-24, saponification value=90-97