Difference between revisions of "Butylated hydroxytoluene"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 9: | Line 9: | ||
[[[SliderGallery rightalign|butylated hydroxytoluene.jpg~Chemical structure]]] | [[[SliderGallery rightalign|butylated hydroxytoluene.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Slightly toxic by inhalation, ingestion and contact. | ||
| + | * Combustible. Flash point = 127C | ||
| + | * Yellows severely with age. | ||
| + | * May decompose to an oily film that migrates from plastics. | ||
| + | * Fisher Scientific: [https://www.fishersci.com/store/msds?partNumber=S25212&productDescription=BUTYL+HYDROXY+TOLUENE+BHT+500G&vendorId=VN00115888&countryCode=US&language=en SDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Soluble in toluene, methanol, ethanol, isopropanol, methyl ethyl ketone, acetone, Cellosolve® and most hydrocarbon solvents. Insoluble in water. | Soluble in toluene, methanol, ethanol, isopropanol, methyl ethyl ketone, acetone, Cellosolve® and most hydrocarbon solvents. Insoluble in water. | ||
| Line 22: | Line 30: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 70 | + | | 70 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.048 | + | | 1.048 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 42: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 265 | + | | 265 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Thomas Gregory, ''The Condensed Chemical Dictionary'', Reinhold Publishing, New York, 3rd ed., 1942 | * Thomas Gregory, ''The Condensed Chemical Dictionary'', Reinhold Publishing, New York, 3rd ed., 1942 | ||
Latest revision as of 13:47, 11 May 2022
Description
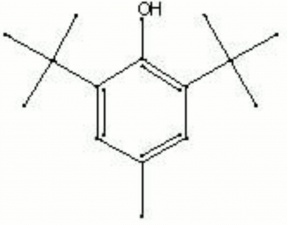
A white, crystalline solid. Butylated hydroxytoluene (BHT) is an antioxidant used in rubber and plastic. It can oxidize to form a dark yellow oily liquid which then migrates to the surface of its plastic or rubber.
Synonyms and Related Terms
BHT; di-tert-butyl-para-cresol; 2,6-Bis(1,1-dimethylethyl)-4-methylphenol; Antrancine 8; Tenox BHT; Ionol CP; Vianol; Dalpac
Risks
- Slightly toxic by inhalation, ingestion and contact.
- Combustible. Flash point = 127C
- Yellows severely with age.
- May decompose to an oily film that migrates from plastics.
- Fisher Scientific: SDS
Physical and Chemical Properties
Soluble in toluene, methanol, ethanol, isopropanol, methyl ethyl ketone, acetone, Cellosolve® and most hydrocarbon solvents. Insoluble in water.
| Composition | [C(CH3)3]2CH3C6H2OH |
|---|---|
| CAS | 128-37-0 |
| Melting Point | 70 C |
| Density | 1.048 g/ml |
| Molecular Weight | mol. wt. = 220.34 |
| Refractive Index | 1.4859 |
| Boiling Point | 265 C |
Resources and Citations
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 274
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: 1521