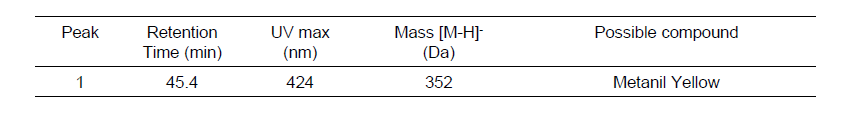Difference between revisions of "CI 13065, Metanil Yellow, Acid Yellow 36, LC"
| (11 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | |||
| + | == Usage History == | ||
| + | |||
| + | [http://cameo.mfa.org/wiki/Metanil_yellow Metanil Yellow] | ||
== Synonyms == | == Synonyms == | ||
| Line 10: | Line 14: | ||
Molecular Weight 375.3768 | Molecular Weight 375.3768 | ||
| + | |||
| + | [[File:CI 13065.PNG|center|frame]] | ||
== Analytical instrumentation and procedures == | == Analytical instrumentation and procedures == | ||
| − | + | Dyes were extracted from textiles (silk or wool) using formic acid/methanol (5:95, v/v). | |
| + | HPLC column: Vydac 214TP52 analytical column (2.1 mm diameterX250 mm; 5-ím particle size). | ||
| − | + | HPLC-DAD-MS instrument: HPLC-DAD-MS analysis was performed with an Agilent 1100 liquid chromatography system consisting of an automatic injector, a gradient pump, a HP series 1100 DAD, and an Agilent series 1100 VL on-line atmospheric pressure ionization electrospray ionization mass spectrometer. | |
| − | + | HPLC gradient profile: Separations were done on a The column was eluted at a flow rate of 0.2 mL/min with a tertiary gradient of water (A),acetonitrile (B), and 1% (v/v) aqueous formic acid (C) with the following elution program: 0 min, 90% A, 5% B, 5% C; 0-55 min, a linear gradient to 35% A, 60% B, 5% C; 55-60 min, a linear gradient elution to 15% A, 80% B, 5% C; 60-62 min, isocratic elution at 15% A, 80% B, 5% C; 62-70 min gradient elution to 90% A, 5% B, 5% C; and reequilibration with the latter solvent for 15 min. The mass spectrometer was run both in the negative ion mode because metanil Yellow is negatively charged without Na+. | |
| + | = Chromatograms = | ||
| − | |||
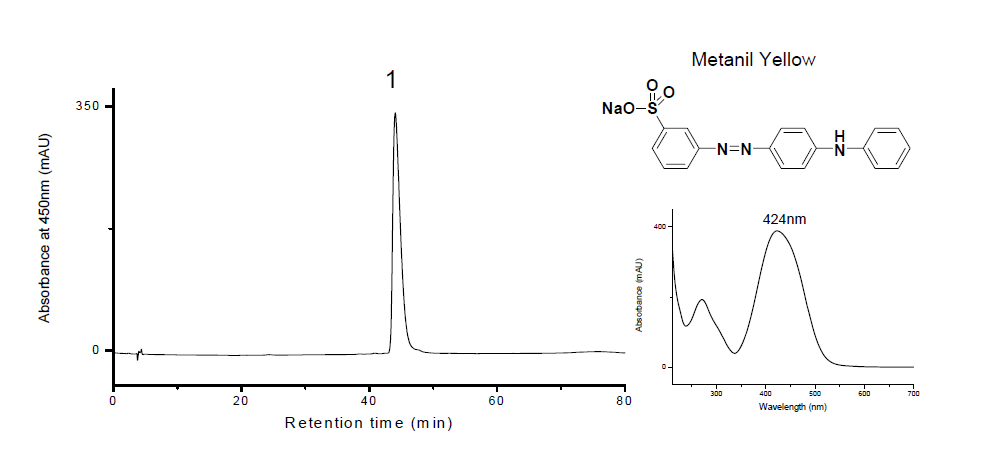
| − | [[File: | + | [[File:Metanil Yellow LC.PNG|center|frame|Absorbance at 450nm (mAU)[1]]] |
| − | |||
| − | + | == Results == | |
| − | + | [[File:Metanil Yellow result.PNG|center|frame|compounds identified]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | | | ||
| − | | | ||
== References == | == References == | ||
| − | + | [1] Zhang, X. Laursen R. and Osipova S. (2005) "Analysis of dyes in some 19th-century Uzbek suzanis"; Dyes in History and Archaeology To be published. | |
[[Category:Dye Analysis]] | [[Category:Dye Analysis]] | ||
[[Category:Reference Materials]] | [[Category:Reference Materials]] | ||
[[Category:Synthetic Dyes]] | [[Category:Synthetic Dyes]] | ||
Latest revision as of 14:03, 3 October 2017
Usage History
Synonyms
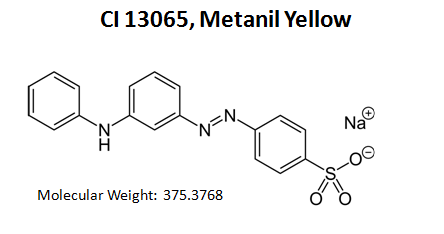
C.I. 13065; C.I. Acid Yellow 36; C.I. Acid Yellow 36 monosodium salt; C.I. Acid Yellow 36, monosodium salt; C.I. Acid Yellow 36, monosodium salt; 3-(4-Anilinophenylazo)benzenesulfonic acid sodium salt; 3-[[4-(Phenylamino)phenyl]azo]benzenesulfonic acid monosodium salt; Acid Yellow 36; metanil yellow (C.I. 13065); sodium 3-(p-anilinophenylazo)benzenesulphonate; Acid Yellow 36 (13065); Acid Golden Yellow G; C.I.Acid Yellow 36; [3-(4-anilinophenyl)azophenyl]sulfanyloxyperoxysodium
Molecular Information
Molecular Formula C18H14N3NaO3S
Molecular Weight 375.3768
Analytical instrumentation and procedures
Dyes were extracted from textiles (silk or wool) using formic acid/methanol (5:95, v/v).
HPLC column: Vydac 214TP52 analytical column (2.1 mm diameterX250 mm; 5-ím particle size).
HPLC-DAD-MS instrument: HPLC-DAD-MS analysis was performed with an Agilent 1100 liquid chromatography system consisting of an automatic injector, a gradient pump, a HP series 1100 DAD, and an Agilent series 1100 VL on-line atmospheric pressure ionization electrospray ionization mass spectrometer.
HPLC gradient profile: Separations were done on a The column was eluted at a flow rate of 0.2 mL/min with a tertiary gradient of water (A),acetonitrile (B), and 1% (v/v) aqueous formic acid (C) with the following elution program: 0 min, 90% A, 5% B, 5% C; 0-55 min, a linear gradient to 35% A, 60% B, 5% C; 55-60 min, a linear gradient elution to 15% A, 80% B, 5% C; 60-62 min, isocratic elution at 15% A, 80% B, 5% C; 62-70 min gradient elution to 90% A, 5% B, 5% C; and reequilibration with the latter solvent for 15 min. The mass spectrometer was run both in the negative ion mode because metanil Yellow is negatively charged without Na+.
Chromatograms
Results
References
[1] Zhang, X. Laursen R. and Osipova S. (2005) "Analysis of dyes in some 19th-century Uzbek suzanis"; Dyes in History and Archaeology To be published.