Difference between revisions of "Calcite"
| Line 40: | Line 40: | ||
| 1.486; 1.658; (1.566) | | 1.486; 1.658; (1.566) | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
[[media:download_file_209.pdf|Properties of Common Abrasives]] | [[media:download_file_209.pdf|Properties of Common Abrasives]] | ||
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
| Line 61: | Line 52: | ||
</gallery> | </gallery> | ||
| + | == Sources Checked for Data in Record == | ||
| + | * R. Gettens, E. West Fitzhugh, R.Feller, "Calcium Carbonate Whites", ''Artists Pigments'', Vol. 2., A. Roy ed. Oxford University Press, Oxford, 1993. | ||
| − | + | * Mineralogy Database: [http://www.webmineral.com/data/Calcite.shtml Calcite] | |
* Thomas Gregory, ''The Condensed Chemical Dictionary'', Reinhold Publishing, New York, 3rd ed., 1942 Comment: Mohs =2 | * Thomas Gregory, ''The Condensed Chemical Dictionary'', Reinhold Publishing, New York, 3rd ed., 1942 Comment: Mohs =2 | ||
| Line 68: | Line 61: | ||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 Comment: Mohs=3 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 Comment: Mohs=3 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "calcite" | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "calcite" [Accessed December 4, 2001] |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| Line 76: | Line 69: | ||
* C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Calcite (Accessed Sept 2 2005; hardness = 3, sp = 2.7 |
* Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | * Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | ||
Revision as of 07:04, 11 August 2020
Description
The most common crystalline form of Calcium carbonate. Calcite is widely distributed throughout the world as Chalk, Limestone, and Marble. Major deposits were formed from ancient sea beds and hard water deposits (e.g., stalactites). Iceland is famous for producing large clear birefractive calcite crystals that are used in optical systems (Iceland spar). In addition to clear colorless crystals, calcite may also appear white or pale shades of other colors depending on the crystal size and the presence of impurities. Marble is limestone that has been metamorphosed to form compact crystals of calcite. Calcite has been gathered or mined since Paleolithic times. In the form of chalk, calcite was powdered for use as a Pigment and as an ingredient in the manufacture of Steel, Cement, and Glass. Limestone and marble are used for sculpture and buildings.
Synonyms and Related Terms
calcium carbonate; marble; limestone; Iceland spar; dogtooth spar; dog-tooth spar; nailhead spar; satin spar; calcareous spar; calc spar; calcspar; calcite (It., Fr., Port.); Kalzit, Calcit (Deut.); calcita (Esp.); calciet (Ned.); calcium salt of carbonic acid; Pigment White 18; CI 7720
Risks
ThermoFisher SDS
Physical and Chemical Properties
Rhombohedral crystal system. Perfect cleavage in three directions. Fracture = conchoidal. High birefringence. Can doubly refract light. Aged surfaces of calcite may fluoresces purple or blue in ultraviolet light; freshly cleaved surfaces are white to yellow. Reacts with acids to evolve carbon dioxide. Luster = vitreous. Streak = white.
| Composition | CaCO3 |
|---|---|
| CAS | 471-34-1 |
| Mohs Hardness | 3.0 |
| Density | 2.71-2.72 |
| Molecular Weight | mol. wt. = 100.09 |
| Refractive Index | 1.486; 1.658; (1.566) |
Comparisons
Properties of Common Abrasives
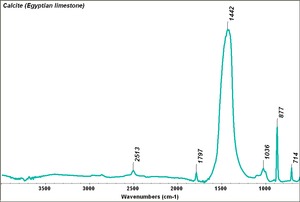
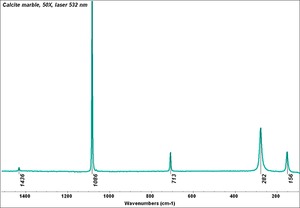
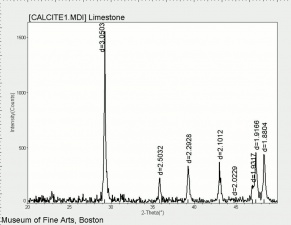
Additional Images
Sources Checked for Data in Record
- R. Gettens, E. West Fitzhugh, R.Feller, "Calcium Carbonate Whites", Artists Pigments, Vol. 2., A. Roy ed. Oxford University Press, Oxford, 1993.
- Mineralogy Database: Calcite
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942 Comment: Mohs =2
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 Comment: Mohs=3
- Encyclopedia Britannica, http://www.britannica.com Comment: "calcite" [Accessed December 4, 2001]
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 138, Mohs=3
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Wikipedia: http://en.wikipedia.org/wiki/Calcite (Accessed Sept 2 2005; hardness = 3, sp = 2.7
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962