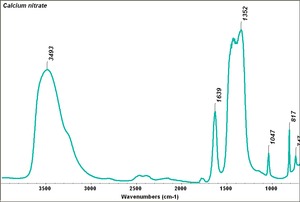
Calcium nitrate
Description
A white, deliquescent solid that is a strong oxidizing agent. Calcium nitrate was first produced commercially in Notodden, Norway in 1905. It is used in pyrotechnics, explosives, and match heads. Calcium nitrate has been identified as a deleterious accretion in wall paintings (Piqué et al 1992). The efflorescence also occurs when manure or other nitrogeneous compounds contact limestone in a dry environment.
Synonyms and Related Terms
calcium dinitrate; lime nitrate; nitrocalcite; lime saltpeter; Norwegian saltpeter (Norgessalpeter); air saltpeter; calciumnitrat (Dan.); Kalksalpeter (Nor.); CalciNit
Other Properties
Soluble in water, methanol, ethanol, acetone. pH = 6.0 (5% solution)
| Composition | Ca(NO3)2 - 4H2O |
|---|---|
| CAS | 10124-37-5 (Anhydrous) 13477-34-4 (Tetrahydrate) |
| Melting Point | 42-45 (hydrated) |
| Density | 1.82 (hydrated); 2.36 (dry) |
| Molecular Weight | mol. wt. = 236.15 |
Hazards and Safety
Fire risk in contact with organic compounds.
Mallinckrodt Baker: MSDS
Additional Information
° F.Piqué, L.Dei, E.Ferroni "Physicochemical Aspects of the Deliquescence of Calcium Nitrate and Its Implications for Wall Painting Conservation" Studies in Conservation 37:217-227, 1992.
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1729
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Calcium_nitrate (Accessed Jan. 15, 2006)
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998