Difference between revisions of "Coconut oil"
Jump to navigation
Jump to search
(username removed) |
|||
| (5 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:Coconutpalmwp2.jpg|thumb|Coconut palm | + | [[File:Coconutpalmwp2.jpg|thumb|Coconut palm ''Cocos nucifera'']] |
| − | |||
| − | ''Cocos nucifera'']] | ||
== Description == | == Description == | ||
| − | A colorless, nondrying oil or white, semisolid fat extracted from the dried meat of the [ | + | A colorless, nondrying oil or white, semisolid fat extracted from the dried meat of the [[coconut]] fruit (copra) obtained from the tropical palm ''Cocus nucifera''. Coconut oil contains the glycerides of [[lauric acid|lauric]] (45.4%), capric (8.4%), myristic (18.0%), [[palmitic acid|palmitic]] (10.5%), [[stearic acid|stearic]] (2.3%), and [[oleic acid|oleic]] (7.5%) acids. It is used commercially to make candy, cosmetics, soaps, emulsions, and leather cleaners. Coconut oil has also been used in gold tooling to adhere gold leaf prior to impressing a heated tool or die (Roberts and Etherington 1982). |
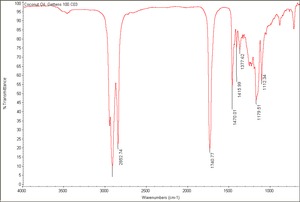
| − | + | [[[SliderGallery rightalign|Coconut Oil, Gettens 100.C03.TIF~FTIR (MFA)]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
''Cocus nucifera''; aceite de coco (Esp.); olio di cocco (It) | ''Cocus nucifera''; aceite de coco (Esp.); olio di cocco (It) | ||
| − | == | + | == Risks == |
| − | + | * Combustible. | |
| + | ==Physical and Chemical Properties== | ||
| − | Soluble in ethanol, ether, chloroform, carbon disulfide. Insoluble in water. | + | * Saponification value = 255-258. |
| + | * Iodine value = 8-9.5. | ||
| + | * Soluble in ethanol, ether, chloroform, carbon disulfide. Insoluble in water. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 22: | Line 23: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 21-25 | + | | 21-25 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.92 | + | | 0.92 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| Line 31: | Line 32: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 210 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2523 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2523 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "coconut palm" | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "coconut palm" [Accessed September 22, 2003]. |
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
Latest revision as of 14:37, 1 July 2022
Description
A colorless, nondrying oil or white, semisolid fat extracted from the dried meat of the Coconut fruit (copra) obtained from the tropical palm Cocus nucifera. Coconut oil contains the glycerides of lauric (45.4%), capric (8.4%), myristic (18.0%), palmitic (10.5%), stearic (2.3%), and oleic (7.5%) acids. It is used commercially to make candy, cosmetics, soaps, emulsions, and leather cleaners. Coconut oil has also been used in gold tooling to adhere gold leaf prior to impressing a heated tool or die (Roberts and Etherington 1982).
Synonyms and Related Terms
Cocus nucifera; aceite de coco (Esp.); olio di cocco (It)
Risks
- Combustible.
Physical and Chemical Properties
- Saponification value = 255-258.
- Iodine value = 8-9.5.
- Soluble in ethanol, ether, chloroform, carbon disulfide. Insoluble in water.
| CAS | 8001-31-8 |
|---|---|
| Melting Point | 21-25 C |
| Density | 0.92 g/ml |
| Refractive Index | 1.4485-1.4495 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 210
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2523
- Encyclopedia Britannica, http://www.britannica.com Comment: "coconut palm" [Accessed September 22, 2003].
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: melting pt.=25.1C, density=0.924, ref. index=1.4463, iodine value = 10.4, saponification value=268