Difference between revisions of "Copper"
| (5 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:17.1970-SC34948.jpg|thumb|]] | + | [[File:17.1970-SC34948.jpg|thumb|Copper bugle<br>MFA# 17.1970]] |
== Description == | == Description == | ||
| − | A reddish-brown, ductile, metallic element. Copper is present in the earth's crust at a concentration of 70 ppm. It occurs as a native metal and as ores of sulfide, sulfate and carbonate ([[azurite]], [[malachite]], [[cuprite]], [[chalcocite]], [[antlerite]], [[chalcopyrite]], etc.). The major mining producers are: Chile, Peru, China, Australia, United States, Russia, Indonesia, Canada, Poland, Kazakhstan. Copper was the first metal used by man, probably from about 8000 BCE, in the regions of Mesopotamia and India. By about 3800 BCE, copper was made into bronze for weapons and knives. Today, copper is one of the most widely used metals. It is high in electrical and thermal conductivity, easily fabricated, ductile, and polishes well. In moist air, copper forms a protective green film of basic carbonate. Metallic copper combines well with other metals to form alloys. The main types of copper alloys are [[brass]] (copper and zinc), low zinc brass, high zinc brass, leaded brass, forging brass, tin brass, [[bronze]] (copper and tin), [[phosphor bronze]], [[silicon bronze]], [[aluminum bronze]], [[nickel silver]] and [[Monel®]]. Copper and its alloys are used for wire, electrical devices, pipes, cooking vessels, ammunition, ornamental trim, roofing, grillwork, coins, musical instruments, jewelry, picture supports, and sculptures. | + | A reddish-brown, ductile, metallic element. Copper is present in the earth's crust at a concentration of 70 ppm. It occurs as a native metal and as ores of sulfide, sulfate and carbonate ([[azurite]], [[malachite]], [[cuprite]], [[chalcocite]], [[antlerite]], [[chalcopyrite]], etc.). The major mining producers are: Chile, Peru, China, Australia, United States, Russia, Indonesia, Canada, Poland, Kazakhstan. Copper was the first metal used by man, probably from about 8000 BCE, in the regions of Mesopotamia and India. By about 3800 BCE, copper was made into bronze for weapons and knives. Today, copper is one of the most widely used metals. It is high in electrical and thermal conductivity, easily fabricated, ductile, and polishes well. In moist air, copper forms a protective green film of basic carbonate. Metallic copper combines well with other metals to form alloys. The main types of copper alloys are [[brass]] (copper and zinc), low zinc brass, high zinc brass, leaded brass, forging brass, tin brass, [[bronze]] (copper and tin), [[phosphor bronze]], [[silicon bronze]], [[aluminum bronze]], [[nickel silver]] and [[Monel|Monel®]]. Copper and its alloys are used for wire, electrical devices, pipes, cooking vessels, ammunition, ornamental trim, roofing, grillwork, coins, musical instruments, jewelry, picture supports, and sculptures. |
| − | [[File:2000.1013-SC9800.jpg|thumb|]] | + | [[File:2000.1013-SC9800.jpg|thumb|Copper tray<br>MFA# 2000.1013]] |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 10: | Line 10: | ||
Cu; aes Cyprium; metal of Cyprus; cuprum (Lat.); Koper (Ned.); cuivre (Fr.); Kupfer (Deut.); rame (It.); cobre (Port., Esp.); Koppar (Sven.); CI 77400; Arwood Copper | Cu; aes Cyprium; metal of Cyprus; cuprum (Lat.); Koper (Ned.); cuivre (Fr.); Kupfer (Deut.); rame (It.); cobre (Port., Esp.); Koppar (Sven.); CI 77400; Arwood Copper | ||
| − | == | + | == Risks== |
| + | |||
| + | * Excess handling of copper salts can cause irritation. | ||
| + | * Food will become toxic when stored in copper containers. | ||
| + | * Copper dust and fumes cause metal fume fever. | ||
| + | * Contact and ingestion may cause irritation and illness. | ||
| + | * ThermoFisher: [https://www.fishersci.com/shop/msdsproxy?productName=AC206530010&productDescription=COPPER SDS] | ||
| + | == Physical and Chemical Properties == | ||
Flame color is green-blue. Soluble in oxidizing acids (nitric, sulfuric, etc.). Slightly soluble in hydrochloric acid, ammonium hydroxide. Ductile, malleable. Luster = metallic. Streak = Copper red. Tarnish = black, blue, green | Flame color is green-blue. Soluble in oxidizing acids (nitric, sulfuric, etc.). Slightly soluble in hydrochloric acid, ammonium hydroxide. Ductile, malleable. Luster = metallic. Streak = Copper red. Tarnish = black, blue, green | ||
| − | [ | + | [[Potassium%20ferrocyanide|Potassium ferrocyanide]] may be used for the colorimetric detection of copper in metal, corrosion products, stains, and pigments. It reacts with copper to form a red-brown cupric ferrocyanide. [[Dithizone|Dithizone]] is also a useful reagent producing a dark yellow residue. |
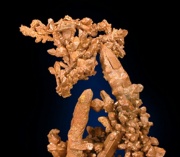
[[File:copperdw.jpg|thumb|Native copper]] | [[File:copperdw.jpg|thumb|Native copper]] | ||
| Line 29: | Line 36: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 1083 | + | | 1083 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 8.95-8.96 | + | | 8.95-8.96 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 38: | Line 45: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 2595 | + | | 2595 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * D.A.Scott, ''Copper and Bronze in Art: Corrosion, Colorants, Conservation,'' Getty Publications, Los Angeles, 2002. | |
| − | + | * J.Waite, "Architectural Metals: Their Deterioration and Stabilization" in ''Preservation and Conservation: Principles and Practice'', S.Timmons (ed.), Preservation Press, Washington DC, 1976, p. 213. | |
| − | + | * Mineralogy Database: [http://www.webmineral.com/data/Copper.shtml Copper] | |
| − | + | * Web Elements: [http://www.webelements.com/webelements/elements/text/Cu/key.html Website] | |
| − | + | * Ancient Trade Routes: [http://www.ancientroute.com/resource/metal/copper.htm Website] | |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 228 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 228 | ||
| Line 77: | Line 76: | ||
* A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 | * A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: copper" | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: copper" [Accessed December 11, 2001]. (color photo); |
* C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | ||
Latest revision as of 13:31, 4 July 2022
Description
A reddish-brown, ductile, metallic element. Copper is present in the earth's crust at a concentration of 70 ppm. It occurs as a native metal and as ores of sulfide, sulfate and carbonate (Azurite, Malachite, Cuprite, Chalcocite, Antlerite, Chalcopyrite, etc.). The major mining producers are: Chile, Peru, China, Australia, United States, Russia, Indonesia, Canada, Poland, Kazakhstan. Copper was the first metal used by man, probably from about 8000 BCE, in the regions of Mesopotamia and India. By about 3800 BCE, copper was made into bronze for weapons and knives. Today, copper is one of the most widely used metals. It is high in electrical and thermal conductivity, easily fabricated, ductile, and polishes well. In moist air, copper forms a protective green film of basic carbonate. Metallic copper combines well with other metals to form alloys. The main types of copper alloys are Brass (copper and zinc), low zinc brass, high zinc brass, leaded brass, forging brass, tin brass, Bronze (copper and tin), Phosphor bronze, Silicon bronze, Aluminum bronze, Nickel silver and Monel®. Copper and its alloys are used for wire, electrical devices, pipes, cooking vessels, ammunition, ornamental trim, roofing, grillwork, coins, musical instruments, jewelry, picture supports, and sculptures.
Synonyms and Related Terms
Cu; aes Cyprium; metal of Cyprus; cuprum (Lat.); Koper (Ned.); cuivre (Fr.); Kupfer (Deut.); rame (It.); cobre (Port., Esp.); Koppar (Sven.); CI 77400; Arwood Copper
Risks
- Excess handling of copper salts can cause irritation.
- Food will become toxic when stored in copper containers.
- Copper dust and fumes cause metal fume fever.
- Contact and ingestion may cause irritation and illness.
- ThermoFisher: SDS
Physical and Chemical Properties
Flame color is green-blue. Soluble in oxidizing acids (nitric, sulfuric, etc.). Slightly soluble in hydrochloric acid, ammonium hydroxide. Ductile, malleable. Luster = metallic. Streak = Copper red. Tarnish = black, blue, green
Potassium ferrocyanide may be used for the colorimetric detection of copper in metal, corrosion products, stains, and pigments. It reacts with copper to form a red-brown cupric ferrocyanide. Dithizone is also a useful reagent producing a dark yellow residue.
| Composition | Cu (atomic no. 29) |
|---|---|
| CAS | 7440-50-8 |
| Mohs Hardness | 2.5 - 3.0 |
| Melting Point | 1083 C |
| Density | 8.95-8.96 g/ml |
| Molecular Weight | atomic wt = 63.546 |
| Boiling Point | 2595 C |
Resources and Citations
- D.A.Scott, Copper and Bronze in Art: Corrosion, Colorants, Conservation, Getty Publications, Los Angeles, 2002.
- J.Waite, "Architectural Metals: Their Deterioration and Stabilization" in Preservation and Conservation: Principles and Practice, S.Timmons (ed.), Preservation Press, Washington DC, 1976, p. 213.
- Mineralogy Database: Copper
- Web Elements: Website
- Ancient Trade Routes: Website
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 228
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- David C. Scott, Metallography and Microstructure of Ancient and Historic Metals, The Getty Conservation Institute, Los Angeles, 1991
- Oppi Untracht, Jewelry Concepts and Technology, Doubleday & Co., Inc., New York City, 1985
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Henry Hodges, Artifacts: An Introduction to Early Materials and Technology, Ronald P. Frye, Kingston, Canada, 1988
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962
- Encyclopedia Britannica, http://www.britannica.com Comment: copper" [Accessed December 11, 2001]. (color photo);
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Copper"
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- George Savage, Art and Antique Restorer's Handbook, Rockliff Publishing Corp, London, 1954