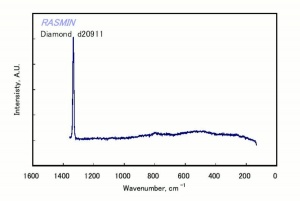
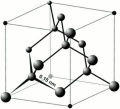
Diamond
Description
A stable, extremely hard, crystalline form of carbon that occurs naturally. Diamonds were known to man prior to 800 BCE from alluvial deposits found in India, Sumatra, and Borneo. They were discovered in Brazil in 1725, in South Africa in 1867, and in Arkansas in 1906. The finest grade diamonds are transparent, brilliant stones that are used as gems; these are primarily mined from the kimberlite pipes in South Africa, and glacial tills of Zaire and Brazil. The largest diamond in the world, the Cullinan diamond, was found in 1905 in South Africa. Named for Sir Thomas Cullinan, it has since been cut into 9 large stones, the largest of which, Great Star of Africa (530.2 carat), is set in the British royal scepter. Diamonds with some imperfections are classified as industrial grade and are used for cutting tools and delicate instruments. These are primarily mined in Australia, Brazil, and South Africa. Abrasive pastes are made from small, imperfectly crystallized pieces called bort (rounded with no distinct cleavage), ballas, and carbonado (black diamond, usually granular with no distinct cleavage). Synthetic diamonds, produced using high heat and pressure, are also used as abrasives. Imitation diamonds have been made from lead glass (diamond paste diamond), quartz rock crystal (rhinestone), cubic oxide zirconia, synthetic rutile, and synthetic spinel.
Synonyms and Related Terms
bort; carbonado; ballas; Cullinan diamond; Great Star of Africa; adamas (ancient Greek); diamant (Ces., Dan., Fr., Ned., Sven.); Diamant (Deut.); diamante (Esp., It., Port.); diament (Pol.)
Other Properties
Hardest natural material. Cubic crystal system. Perfect cleavage in four directions.
Luster = greasy to adamantine. Streak = colorless.
Fluorescence: many diamonds fluoresce pale colors. Some may phophoresce.
| Mohs Hardness | 10 |
|---|---|
| Melting Point | 3700 |
| Density | 3.51-3.53 |
| Refractive Index | 2.417-2.4195 |
| Boiling Point | 4200 |
Hazards and Safety
No significant hazards.
Additional Information
Mineralogy Database: Diamond
Comparisons
Properties of Common Abrasives
Properties of Common Gemstones
Natural and Simulated Diamonds
Additional Images
Authority
- Jack Odgen, Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- R.F.Symmes, T.T.Harding, Paul Taylor, R.F.Symmes, T.T.Harding, Paul Taylor, Rocks, Fossils and Gems, DK Publishing, Inc., New York City, 1997
- Encyclopedia Britannica, http://www.britannica.com Comment: "diamond" Encyclopdia Britannica [Accessed March 4, 2002].
- Website address 1, Website address 1 Comment: http://www.geo.utexas.edu/courses/347k/redesign/gem_notes/Diamond/diamond_triple_frame.htm (flourescence information)
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Diamond (Accessed Mar. 1, 2006) (synonyms)
- Yasukazu Suwa, Yasukazu Suwa, Gemstones: Quality and Value, Volume 1, Sekai Bunka Publishing Inc., Tokyo, 1999 Comment: RI=2.417; Specific gravity=3.52;
- Michael O'Donoghue and Louise Joyner, Michael O'Donoghue and Louise Joyner, Identification of Gemstones, Butterworth-Heinemann, Oxford, 2003 Comment: RI=2.417; Specific gravity=3.52; dispersion=0.44
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- G.S.Brady, G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Michael McCann, Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- R.M.Organ, R.M.Organ, Design for Scientific Conservation of Antiquities, Smithsonian Institution, Washington DC, 1968
- Thomas B. Brill, Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=3.01-3.52