Difference between revisions of "Dolomite"
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:01.7287-CR1881-d1.jpg|thumb|]] | + | [[File:01.7287-CR1881-d1.jpg|thumb|Egyptian vessel<br>MFA# 01.7287]] |
== Description == | == Description == | ||
| Line 10: | Line 10: | ||
calcium magnesium carbonate; magnesium calcium carbonate; Dolomit (Deut.); dolomita (Esp.); dolomie (Fr.); dolomiet (Ned.); dolomite (Port.); bitter spar; pearl spar; dolomitic marble | calcium magnesium carbonate; magnesium calcium carbonate; Dolomit (Deut.); dolomita (Esp.); dolomie (Fr.); dolomiet (Ned.); dolomite (Port.); bitter spar; pearl spar; dolomitic marble | ||
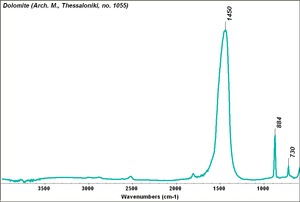
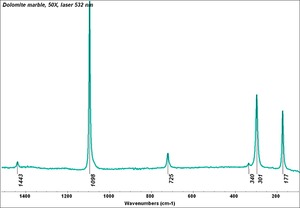
| − | [[[SliderGallery rightalign|Dolomite.TIF~FTIR (MFA)|Dolomite marble, 50X, laser 532 nm copy.tif | + | == Physical and Chemical Properties == |
| − | + | [[[SliderGallery rightalign|Dolomite.TIF~FTIR (MFA)|Dolomite marble, 50X, laser 532 nm copy.tif~Raman (MFA)]]] | |
| − | + | * Hexagonal crystal systems with rhombohedral habits. | |
| − | + | * Cleavage is parallel to the rhombohedron; perfect in three directions. | |
| − | Hexagonal crystal systems with rhombohedral habits. Cleavage is parallel to the rhombohedron; perfect in three directions. | + | * Fracture = subconchoidal. |
| − | + | * Luster = vitreous to pearly. | |
| − | Fracture = subconchoidal. Luster = vitreous to pearly. Streak = white. | + | * Streak = white. |
| − | + | * Under cross polars has high birefringence with strong interference colors | |
| − | Under cross polars has high birefringence with strong interference colors | + | * Dissolves slowly in dilute cold hydrochloric acid with effervescence. |
| − | |||
| − | Dissolves slowly in dilute cold hydrochloric acid with effervescence. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 31: | Line 29: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.8-2.9 | + | | 2.8-2.9 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| w=1.679; e=1.500 | | w=1.679; e=1.500 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
| Line 49: | Line 43: | ||
</gallery> | </gallery> | ||
| + | ==Resources and Citations== | ||
| − | + | * Mineralogy Database: [http://www.webmineral.com/data/Dolomite.shtml Dolomite] | |
| − | * | + | * José Delgado Rodrigues, LNEC, Submitted information, 2009. |
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive index: w=1.679; e=1.500 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive index: w=1.679; e=1.500 | ||
| Line 60: | Line 55: | ||
* Anne Grimmer, Glossary of Building Stone Terms, ''A Glossary of Historic Masonry Deterioration Problems and Preservation Treatments'', National Park Service, Washington DC, 1984 | * Anne Grimmer, Glossary of Building Stone Terms, ''A Glossary of Historic Masonry Deterioration Problems and Preservation Treatments'', National Park Service, Washington DC, 1984 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "dolomite" | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "dolomite" [Accessed December 4, 2001] |
* C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Dolomite (Accessed Sept. 7, 2005) |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
Latest revision as of 14:39, 22 July 2022
Description
A pearly sedimentary mineral composed of calcium-magnesium carbonate. that was first described in 1791 by the French naturalist Deodat de Dolomieu in the Dolomite mountains in Italy. Domolite rock is hard, crystalline, carbonaceous stone with high percentage (90 % to 100 %) of the mineral dolomite. It is found in ledge formations throughout Europe (Saxony, Switzerland, Italy, France, Spain, Greece), South America (Brazil) and the United States (Vermont, New York, Pennsylvania, North Carolina, Illinois, Missouri). Although usually a translucent white in color, the mineral dolomite varies widely to yellow, pink, green, brown and gray. Iron is often a minor component replacing some of the Magnesium. Dolomite rock has been and is currently used as a building stone, in furnace linings, in ceramics, and as a Filler in Paper. Under high temperatures and pressures, dolomite is metamorphosed into Dolomitic marble.
Synonyms and Related Terms
calcium magnesium carbonate; magnesium calcium carbonate; Dolomit (Deut.); dolomita (Esp.); dolomie (Fr.); dolomiet (Ned.); dolomite (Port.); bitter spar; pearl spar; dolomitic marble
Physical and Chemical Properties
- Hexagonal crystal systems with rhombohedral habits.
- Cleavage is parallel to the rhombohedron; perfect in three directions.
- Fracture = subconchoidal.
- Luster = vitreous to pearly.
- Streak = white.
- Under cross polars has high birefringence with strong interference colors
- Dissolves slowly in dilute cold hydrochloric acid with effervescence.
| Composition | CaMg(CO3)2 |
|---|---|
| Mohs Hardness | 3.5 - 4.0 |
| Density | 2.8-2.9 g/ml |
| Refractive Index | w=1.679; e=1.500 |
Additional Images
Resources and Citations
- Mineralogy Database: Dolomite
- José Delgado Rodrigues, LNEC, Submitted information, 2009.
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive index: w=1.679; e=1.500
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Anne Grimmer, Glossary of Building Stone Terms, A Glossary of Historic Masonry Deterioration Problems and Preservation Treatments, National Park Service, Washington DC, 1984
- Encyclopedia Britannica, http://www.britannica.com Comment: "dolomite" [Accessed December 4, 2001]
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Wikipedia: http://en.wikipedia.org/wiki/Dolomite (Accessed Sept. 7, 2005)
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 273
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985