Difference between revisions of "EDTA"
| (One intermediate revision by one other user not shown) | |||
| Line 2: | Line 2: | ||
An acronym for ethylenediamine tetraacetic acid and many of the metal salts derived from this acid. The metal salts, such as [[calcium]], [[sodium]], etc., are generically called edates. EDTA has colorless crystals that are slightly soluble in water and insoluble in organic solvents. EDTA is a strong chelating agent used to remove metallic salts from solutions. The use of EDTA has had some success in cleaning stains and crusts from stone. Its ability to selectively remove stains is determined by the number and type of attached metal salts. For example, disodium EDTA is acidic and quickly solubilizes [[calcite]], while tetrasodium EDTA is basic and better dissolves [[gypsum]] (Thorn, 1993). EDTA is also used in detergents, soaps, shampoos, textiles, and food. | An acronym for ethylenediamine tetraacetic acid and many of the metal salts derived from this acid. The metal salts, such as [[calcium]], [[sodium]], etc., are generically called edates. EDTA has colorless crystals that are slightly soluble in water and insoluble in organic solvents. EDTA is a strong chelating agent used to remove metallic salts from solutions. The use of EDTA has had some success in cleaning stains and crusts from stone. Its ability to selectively remove stains is determined by the number and type of attached metal salts. For example, disodium EDTA is acidic and quickly solubilizes [[calcite]], while tetrasodium EDTA is basic and better dissolves [[gypsum]] (Thorn, 1993). EDTA is also used in detergents, soaps, shampoos, textiles, and food. | ||
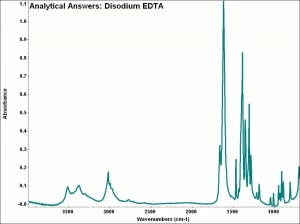
| − | + | [[[SliderGallery rightalign|aaiNA2EDTA.jpg~FTIR|EDTA.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
ethylenediamine tetraacetic acid; n,n'-1,2-ethanediylbis(n-carboxymethyl)-glycine; edetic acid; sodium EDTA | ethylenediamine tetraacetic acid; n,n'-1,2-ethanediylbis(n-carboxymethyl)-glycine; edetic acid; sodium EDTA | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Contact with concentrated EDTA may cause skin and eye irritation. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=BP118500&productDescription=EDTA+%28FREE+ACID%29+500G&vendorId=VN00033897&countryCode=US&language=en SDS} | ||
| − | == | + | ==Physical and Chemical Properties== |
Slightly soluble in water. Insoluble in organic solvents. | Slightly soluble in water. Insoluble in organic solvents. | ||
| Line 25: | Line 28: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * A.Thorn "The Impact of Disodium EDTA on Stone" ICOM Preprints, Washington DC, 1993, p. 357-363. | |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 45: | Line 40: | ||
* ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 3490 | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 3490 | ||
| − | * | + | * Conservation termlist at www.hants.org.uk/museums |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 11:07, 1 August 2022
Description
An acronym for ethylenediamine tetraacetic acid and many of the metal salts derived from this acid. The metal salts, such as Calcium, Sodium, etc., are generically called edates. EDTA has colorless crystals that are slightly soluble in water and insoluble in organic solvents. EDTA is a strong chelating agent used to remove metallic salts from solutions. The use of EDTA has had some success in cleaning stains and crusts from stone. Its ability to selectively remove stains is determined by the number and type of attached metal salts. For example, disodium EDTA is acidic and quickly solubilizes Calcite, while tetrasodium EDTA is basic and better dissolves Gypsum (Thorn, 1993). EDTA is also used in detergents, soaps, shampoos, textiles, and food.
Synonyms and Related Terms
ethylenediamine tetraacetic acid; n,n'-1,2-ethanediylbis(n-carboxymethyl)-glycine; edetic acid; sodium EDTA
Risks
- Contact with concentrated EDTA may cause skin and eye irritation.
- ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=BP118500&productDescription=EDTA+%28FREE+ACID%29+500G&vendorId=VN00033897&countryCode=US&language=en SDS}
Physical and Chemical Properties
Slightly soluble in water. Insoluble in organic solvents.
| Composition | C10H16N2O8 |
|---|---|
| CAS | 60-00-4 |
| Molecular Weight | mol. wt. = 292.2 |
Resources and Citations
- A.Thorn "The Impact of Disodium EDTA on Stone" ICOM Preprints, Washington DC, 1993, p. 357-363.
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, Notes for Workshop on New Methods in the Cleaning of Paintings, J.Paul Getty Trust, Los Angeles, 1990
- Marie Svoboda, Conservation Survey Index, unpublished, 1997
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 3490
- Conservation termlist at www.hants.org.uk/museums