Difference between revisions of "Epichlorohydrin"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 2: | Line 2: | ||
A clear, colorless liquid with a chloroform-like odor. Epichlorohydrin is an epoxide compound that is volatile, highly reactive and unstable. Most commonly, epichlorohydrin is combined with [[Bisphenol A]] to form [[epoxy]] resins. It is also used as a [[solvent]] for natural and synthetic resins, gums, cellulose esters and ethers in paint, varnish, and nail enamel formulations. Epichlorohydrin is used by the paper industry as an additive to paper pulp because it crosslinks on drying to form a high wet-strength paper. In textile applications, epichlorohydrin is used to modify wool by making it more durable and insect resistant. Epichlorohydrin has also been used as an [[insecticide]]. | A clear, colorless liquid with a chloroform-like odor. Epichlorohydrin is an epoxide compound that is volatile, highly reactive and unstable. Most commonly, epichlorohydrin is combined with [[Bisphenol A]] to form [[epoxy]] resins. It is also used as a [[solvent]] for natural and synthetic resins, gums, cellulose esters and ethers in paint, varnish, and nail enamel formulations. Epichlorohydrin is used by the paper industry as an additive to paper pulp because it crosslinks on drying to form a high wet-strength paper. In textile applications, epichlorohydrin is used to modify wool by making it more durable and insect resistant. Epichlorohydrin has also been used as an [[insecticide]]. | ||
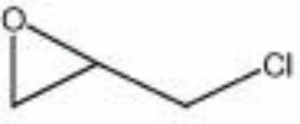
| + | [[[SliderGallery rightalign|epichlorohydrin.jpg~Chemical structure]]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 7: | Line 8: | ||
chloropropylene oxide; chloromethyloxirane; chlorohydrin; 1-chloro-2,3-epoxypropane | chloropropylene oxide; chloromethyloxirane; chlorohydrin; 1-chloro-2,3-epoxypropane | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Toxic by inhalation, ingestion and skin absorption. | ||
| + | * Overexposure may cause nausea, severe pain, respiratory distress and membrane irritation. | ||
| + | * Human carcinogen. | ||
| + | * Sigma Aldrich: [https://www.sigmaaldrich.com/US/en/product/aldrich/e1055 SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Miscible with ethanol, ether, chloroform, trichloroethylene, carbon tetrachloride. Immiscible with water, petroleum hydrocarbons. | Miscible with ethanol, ether, chloroform, trichloroethylene, carbon tetrachloride. Immiscible with water, petroleum hydrocarbons. | ||
| Line 22: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -25.6 | + | | -25.6 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.18 | + | | 1.18 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 40: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 117.9 | + | | 117.9 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 59: | Line 58: | ||
* Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 | * Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 | ||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 08:36, 2 August 2022
Description
A clear, colorless liquid with a chloroform-like odor. Epichlorohydrin is an epoxide compound that is volatile, highly reactive and unstable. Most commonly, epichlorohydrin is combined with Bisphenol A to form Epoxy resins. It is also used as a Solvent for natural and synthetic resins, gums, cellulose esters and ethers in paint, varnish, and nail enamel formulations. Epichlorohydrin is used by the paper industry as an additive to paper pulp because it crosslinks on drying to form a high wet-strength paper. In textile applications, epichlorohydrin is used to modify wool by making it more durable and insect resistant. Epichlorohydrin has also been used as an Insecticide.
Synonyms and Related Terms
chloropropylene oxide; chloromethyloxirane; chlorohydrin; 1-chloro-2,3-epoxypropane
Risks
- Toxic by inhalation, ingestion and skin absorption.
- Overexposure may cause nausea, severe pain, respiratory distress and membrane irritation.
- Human carcinogen.
- Sigma Aldrich: SDS
Physical and Chemical Properties
Miscible with ethanol, ether, chloroform, trichloroethylene, carbon tetrachloride. Immiscible with water, petroleum hydrocarbons.
| Composition | C3H5OCl |
|---|---|
| CAS | 106-89-8 |
| Melting Point | -25.6 C |
| Density | 1.18 g/ml |
| Molecular Weight | mol. wt. = 92.5 |
| Refractive Index | 1.436 |
| Boiling Point | 117.9 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3648
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.436
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000