Difference between revisions of "Hansa red"
Jump to navigation
Jump to search
| (4 intermediate revisions by one other user not shown) | |||
| Line 4: | Line 4: | ||
See also [[Hansa]]. | See also [[Hansa]]. | ||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
toluidine red; Pigment Red 3; CI 12120 | toluidine red; Pigment Red 3; CI 12120 | ||
| − | |||
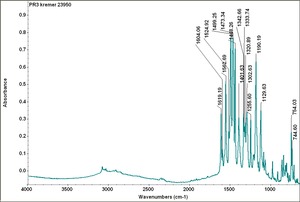
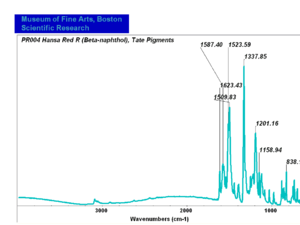
[[[SliderGallery rightalign|PR3 kremer 23950.TIF~FTIR PR3(MFA)|PR004 Hansa Red R (Tate).PNG~FTIR PR4(MFA)]]] | [[[SliderGallery rightalign|PR3 kremer 23950.TIF~FTIR PR3(MFA)|PR004 Hansa Red R (Tate).PNG~FTIR PR4(MFA)]]] | ||
== Comparisons == | == Comparisons == | ||
| − | |||
{| class="wikitable" | {| class="wikitable" | ||
! Pigment number !! Manufacture !! Pigment name !! Manufacture CI number !! Comments | ! Pigment number !! Manufacture !! Pigment name !! Manufacture CI number !! Comments | ||
|- | |- | ||
| − | | PR003 || Kremer || | + | | PR003 || Kremer || studio red, helio || 23950 || |
| − | |||
| − | |||
|- | |- | ||
| + | | PR004 || unknown|| Hansa red R (beta-naphthol) || unknown || sample from Tate Collection | ||
|} | |} | ||
| − | == | + | == Risks == |
| − | + | * Ingestion can cause cyanosis. | |
| + | * Suspected carcinogen. | ||
| − | == | + | ==Physical and Chemical Properties== |
| − | + | Soluble in many organic solvents. Resistant to acid, alkali and soap. | |
| − | == | + | ==Resources and Citations== |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 14:38, 30 August 2022
Description
Originally a Hoechst AG trademark for a line of bright, transparent red synthetic organic pigments. Hansa reds, also called toluidine reds, are based on the reaction of beta-naphthol with 2-nitro-4-toluidine. Toluidine red was first synthesized in 1905. In general, toluidine reds have fair lightfastness and weather resistance, and they have a tendency to bleed. They are used in industrial coatings for air-dried and baked enamels and auto finishes. Toluidine reds are also used in wax crayons, pastels and watercolors.
See also Hansa.
Synonyms and Related Terms
toluidine red; Pigment Red 3; CI 12120
Comparisons
| Pigment number | Manufacture | Pigment name | Manufacture CI number | Comments |
|---|---|---|---|---|
| PR003 | Kremer | studio red, helio | 23950 | |
| PR004 | unknown | Hansa red R (beta-naphthol) | unknown | sample from Tate Collection |
Risks
- Ingestion can cause cyanosis.
- Suspected carcinogen.
Physical and Chemical Properties
Soluble in many organic solvents. Resistant to acid, alkali and soap.
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Monona Rossol, The Artist's Complete Health and Safety Guide, Allworth Press, New York, 1994
- B. Berrie, S.Q. Lomax, 'Azo Pigments: Their History, Synthesis, Properties and Use in Artists' Materials', Studies in the History of Art , National Gallery of Art, Washington DC, No. 57, 1997