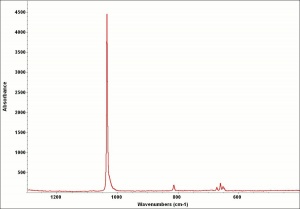
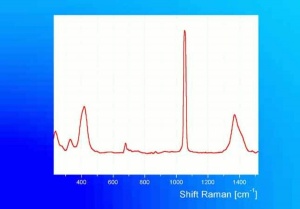
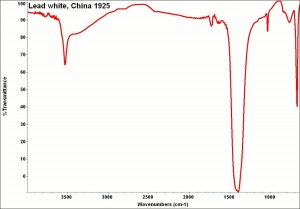
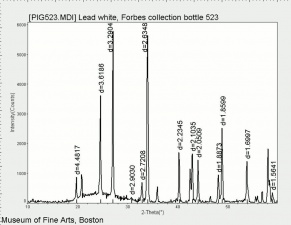
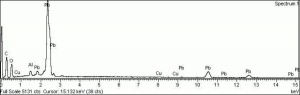
Lead carbonate, basic
Description
A white amorphous powder that was the primary white pigment (Lead white) for oil paint and ceramic glazes from ancient times until the 20th century. Basic lead carbonate is rarely found as the natural mineral, hydrocerussite. Instead it has been made since early times by placing Lead plates in the presence of Acetic acid (vinegar) fumes. This produces Lead acetate which then absorbs Carbon dioxide from the atmosphere (or another source) and converts to basic lead carbonate. Lead white is a dense, opaque pigment that was mainly used in drying oils where it acts as a siccative. It has also been found in Egg tempera, Glue tempera, and Gum tempera, but it was not considered suitable for buon fresco techniques. Although basic lead carbonate has been replaced as primary paint pigment by Zinc white and Titanium white, it can still be found in some exterior paints and ceramic glazes. In the United States, its use in interior paints has been restricted since the 1950s and prohibited since 1978.
Synonyms and Related Terms
basic lead carbonate; basic white lead; lead subcarbonate; lead white; hydrocerussite; lead flake; flake white; Cremnitz white; white lead; Krems white; silver white; Dutch white lead; French white; Vienna white; Flemish white; London white; Roman white; Pigment White 1; blanc de plomb (Fr.); Bleiweiss (Deut.); bianco di piombo (It.); plomo blanco (Esp.)
Risks
- Toxic by inhalation or ingestion.
- Skin contact may cause irritation or ulcers.
- Carcinogen, teratogen, suspected mutagen.
- Darkens in the presence of sulfur fumes.
- Susceptible to biological deterioration.
- ThermoFisher: SDS
Physical and Chemical Properties
- Soluble in acids. Insoluble in water and ethanol.
- Fluoresces reddish purple.
- Pigment has fine, fairly uniform, rounded tabular particles.
- High birefringence under cross polars with 3rd or 4th order interference colors. Complete extinction for single particles, aggregates appear to twinkle
| Composition | 2PbCO3.Pb(OH)2 |
|---|---|
| CAS | 1319-46-6 |
| Density | 6.70-6.86 g/ml |
| Molecular Weight | mol. wt. = 775.62 |
| Refractive Index | e=1.94; w=2.09 |
Comparisons
Characteristics of Common White Pigments
Resources and Citations
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Vol. 2, page 303
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 6.70 and ref.index.= 1.94;2.09
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Artists' Pigments: A Handbook of their History and Characteristics, Ashok Roy (ed.), National Gallery of Art, Washington DC, Vol. 2, 1993 Comment: R.J.Gettens, H. Kuhn, and W.T. Chase, "Lead White", ref. index = 1.94; 2.09
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: ref. index = 1.94; 2.09
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- MSDS Sheet Comment: Baker MSDS Spec gravity = 6.14
- Website address 1 Comment: Pigments Through the Ages - http://webexhibits.org/pigments/indiv/overview/leadwhite.html refractive index: Uniaxial (-), e = 1.94, w = 2.09