Difference between revisions of "Methanol"
(Created) |
(Moved main article from Methyl alcohol) |
||
| Line 1: | Line 1: | ||
| + | == Description == | ||
| + | |||
| + | A clear, colorless, slightly volatile solvent with a slight alcoholic smell. Methanol, commonly known as methyl alcohol, was discovered in 1661 by R. Boyle. It is made by the destructive distillation of [http://cameo.mfa.org/materials/fullrecord.asp?name=wood wood], peat, and [http://cameo.mfa.org/materials/fullrecord.asp?name=lignite lignite] as well as by the partial oxidation of natural gas hydrocarbons. Methanol is highly polar and is usually a better solvent than [http://cameo.mfa.org/materials/fullrecord.asp?name=ethyl%20alcohol ethanol]. It is used in the manufacture of several organic compounds. Methanol is also used as a solvent for [http://cameo.mfa.org/materials/fullrecord.asp?name=lacquer%2C%20synthetic lacquers], [http://cameo.mfa.org/materials/fullrecord.asp?name=shellac shellac], [http://cameo.mfa.org/materials/fullrecord.asp?name=rosin rosin], [http://cameo.mfa.org/materials/fullrecord.asp?name=dye dyes], [http://cameo.mfa.org/materials/fullrecord.asp?name=oil oils], [http://cameo.mfa.org/materials/fullrecord.asp?name=paint%20remover paint removers], and degreasing compounds. Because of its toxicity, methanol is used to denature ethanol. | ||
| + | |||
| + | == Synonyms and Related Terms == | ||
| + | |||
| + | methanol (IUPAC); wood alcohol; wood spirits; pyroxylic spirit; wood naphtha; methyl hydrate; methylic alcohol; Columbian spirits; carbinol | ||
| + | |||
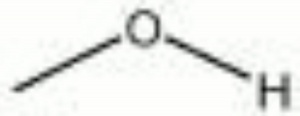
| + | [[[SliderGallery rightalign|methyl alcohol.jpg~Chemical structure]]] | ||
| + | |||
| + | == Other Properties == | ||
| + | |||
| + | Miscible with water, ethanol, ether, benzene, ketones and most other organic solvents. Methanol burns with a pale blue flame. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! scope="row"| Composition | ||
| + | | CH3OH | ||
| + | |- | ||
| + | ! scope="row"| CAS | ||
| + | | 67-56-1 | ||
| + | |- | ||
| + | ! scope="row"| Melting Point | ||
| + | | -97.8 | ||
| + | |- | ||
| + | ! scope="row"| Density | ||
| + | | 0.7866 | ||
| + | |- | ||
| + | ! scope="row"| Molecular Weight | ||
| + | | mol. wt.=32.04 | ||
| + | |- | ||
| + | ! scope="row"| Refractive Index | ||
| + | | 1.326-1.329 | ||
| + | |- | ||
| + | ! scope="row"| Boiling Point | ||
| + | | 64.7 | ||
| + | |} | ||
| + | |||
| + | == Hazards and Safety == | ||
| + | |||
| + | Flammable. Flash point = 12 C (54 F) | ||
| + | |||
| + | Dangerous fire risk. Forms an explosive mixture with air. | ||
| + | |||
| + | Toxic by ingestion and inhalation. Ingestion of small amounts will cause blindness. | ||
| + | |||
| + | LINK: [http://www.cdc.gov/niosh/ipcsneng/neng0057.html International Chemical Safety Card] | ||
| + | |||
| + | == Authority == | ||
| + | |||
| + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| + | |||
| + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 505 | ||
| + | |||
| + | * Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 | ||
| + | |||
| + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| + | |||
| + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| + | |||
| + | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | ||
| + | |||
| + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| + | |||
| + | * Kurt Wehlte, ''The Materials and Techniques of Painting'', Van Nostrand Reinhold Co., New York, 1975 | ||
| + | |||
| + | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6024; ref. index = 1.329 | ||
| + | |||
| + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Methanol." Encyclopædia Britannica. 7 July 2004 . | ||
| + | |||
| + | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| + | |||
| + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| + | |||
| + | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| + | |||
| + | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.326 | ||
| + | |||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 03:39, 10 February 2016
Description
A clear, colorless, slightly volatile solvent with a slight alcoholic smell. Methanol, commonly known as methyl alcohol, was discovered in 1661 by R. Boyle. It is made by the destructive distillation of wood, peat, and lignite as well as by the partial oxidation of natural gas hydrocarbons. Methanol is highly polar and is usually a better solvent than ethanol. It is used in the manufacture of several organic compounds. Methanol is also used as a solvent for lacquers, shellac, rosin, dyes, oils, paint removers, and degreasing compounds. Because of its toxicity, methanol is used to denature ethanol.
Synonyms and Related Terms
methanol (IUPAC); wood alcohol; wood spirits; pyroxylic spirit; wood naphtha; methyl hydrate; methylic alcohol; Columbian spirits; carbinol
Other Properties
Miscible with water, ethanol, ether, benzene, ketones and most other organic solvents. Methanol burns with a pale blue flame.
| Composition | CH3OH |
|---|---|
| CAS | 67-56-1 |
| Melting Point | -97.8 |
| Density | 0.7866 |
| Molecular Weight | mol. wt.=32.04 |
| Refractive Index | 1.326-1.329 |
| Boiling Point | 64.7 |
Hazards and Safety
Flammable. Flash point = 12 C (54 F)
Dangerous fire risk. Forms an explosive mixture with air.
Toxic by ingestion and inhalation. Ingestion of small amounts will cause blindness.
LINK: International Chemical Safety Card
Authority
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 505
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Kurt Wehlte, The Materials and Techniques of Painting, Van Nostrand Reinhold Co., New York, 1975
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6024; ref. index = 1.329
- Encyclopedia Britannica, http://www.britannica.com Comment: "Methanol." Encyclopædia Britannica. 7 July 2004 .
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.326