Difference between revisions of "Morin"
(username removed) |
(username removed) |
||
| Line 38: | Line 38: | ||
== Authority == | == Authority == | ||
| − | * | + | * F. Crace-Calvert, ''Dyeing and Calico Printing'', Palmer & Howe, London, 1876 |
| − | * | + | * Judith Hofenk-de Graaff, ''Natural Dyestuffs: Origin, Chemical Constitution, Identification'', Central Research Laboratory for Objects of Art and Science, Amsterdam, 1969 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6352 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6352 | ||
Revision as of 07:44, 24 July 2013
Description
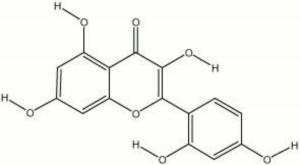
A colorant found in old fustic, Cuba wood, yellow brazilwood, jakwood, and Osage orange tree. Morin crystallizes from ethanol to form colorless needles. It turns an intense yellow in alkaline solutions, but will slowly turn brown with exposure to air. Morin is used as a dye for wools: with chromium it gives an olive green, with aluminum and tin, it is yellow, and with iron, morin is a deep olive brown. It is also used as a spot test reagent for salts of aluminum, beryllium, zinc, gallium, indium, and selenium.
Synonyms and Related Terms
2',3,4,5,7-pentahydroxyflavone; Natural Yellow 8; Natural Yellow 11; CI 75660; morina (Esp., Port.); morin hydrate; moric acid
Other Properties
Soluble in ethanol, alkalis, acetic acid. Slightly soluble in water.
Soluble in sulfuric acid forming a solution with green fluorescence.
| Composition | C15H10O7-2H2O |
|---|---|
| CAS | 480-16-0 |
| Melting Point | 285 |
| Molecular Weight | mol. wt. = 302.23 |
Hazards and Safety
Combustible. Contact may cause irritation.
Fisher Scientific: MSDS
Authority
- F. Crace-Calvert, Dyeing and Calico Printing, Palmer & Howe, London, 1876
- Judith Hofenk-de Graaff, Natural Dyestuffs: Origin, Chemical Constitution, Identification, Central Research Laboratory for Objects of Art and Science, Amsterdam, 1969
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6352