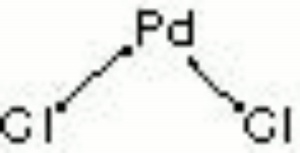Difference between revisions of "Palladium chloride"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 9: | Line 9: | ||
[[[SliderGallery rightalign|palladium chloride.jpg~Chemical structure]]] | [[[SliderGallery rightalign|palladium chloride.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Suspected carcinogen. | ||
| + | * Hygroscopic. | ||
| + | * Contact may cause irritation. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/01153.htm MSDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Soluble in water, hydrochloric acid, ethanol, acetone. | Soluble in water, hydrochloric acid, ethanol, acetone. | ||
| Line 22: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 675 | + | | 675 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 4.0 | + | | 4.0 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 38: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * R. Waller, K.Andrew, J.Tetreault, "Survey of Gaseous Pollutant Concentration Distributions in Mineral Collections" Collection Forum, 14(1-2):1-32, 2000. | |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Revision as of 12:32, 10 August 2022
Description
Dark brown deliquescent powder that is used as a brightener in metal coatings. Palladium chloride is also used in indelible inks, photography, and glassmaking. Solutions of palladium chloride have been used to make indicator papers that are sensitive to Mercury vapors (Waller et al 2000).
Synonyms and Related Terms
palladous chloride; palladium dichloride; palladium (II) chloride
Risks
- Suspected carcinogen.
- Hygroscopic.
- Contact may cause irritation.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in water, hydrochloric acid, ethanol, acetone.
| Composition | PdCl2 |
|---|---|
| CAS | 7647-10-1 |
| Melting Point | 675 C |
| Density | 4.0 g/ml |
| Molecular Weight | mol. wt. = 177.306 |
Resources and Citations
- R. Waller, K.Andrew, J.Tetreault, "Survey of Gaseous Pollutant Concentration Distributions in Mineral Collections" Collection Forum, 14(1-2):1-32, 2000.
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979