Difference between revisions of "Phenolphthalein"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 2: | Line 2: | ||
Pale yellow powder that is used as a pH indicator. Phenolphthalein is a triphenylmethane salt whose three aromatic rings produce a red chromophore in basic and neutral solutions. The conjugation is disrupted in acidic solutions resulting in a clear solution. Thus phenolphthalein is an effective acid base indicator for titrations of mineral acids, organic acids and alkalis. It is prepared as a 1% solution in ethanol. In solutions with a pH below 8.5, phenolphthalein is colorless and solutions above pH 9 are red. | Pale yellow powder that is used as a pH indicator. Phenolphthalein is a triphenylmethane salt whose three aromatic rings produce a red chromophore in basic and neutral solutions. The conjugation is disrupted in acidic solutions resulting in a clear solution. Thus phenolphthalein is an effective acid base indicator for titrations of mineral acids, organic acids and alkalis. It is prepared as a 1% solution in ethanol. In solutions with a pH below 8.5, phenolphthalein is colorless and solutions above pH 9 are red. | ||
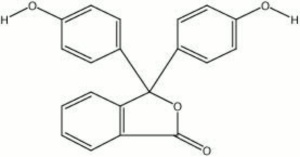
| − | + | [[[SliderGallery rightalign|phenolphthalein.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
3,3-bis(p-hydroxyphenyl)phthalide; 3,3-bis(4-hydroxyphenyl)-1(3H)-isobenzofuranore | 3,3-bis(p-hydroxyphenyl)phthalide; 3,3-bis(4-hydroxyphenyl)-1(3H)-isobenzofuranore | ||
| − | + | == Risks == | |
| − | == | + | * Toxic by ingestion, even small amounts will cause illness. |
| + | * Suspected carcinogen. | ||
| + | * Contact may cause irritation. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=P79500&productDescription=PHENOLPHTHALEIN+CERT+ACS+500G&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in ethanol, ether and alkalis. Insoluble in water. | Soluble in ethanol, ether and alkalis. Insoluble in water. | ||
| Line 24: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 258-262 | + | | 258-262 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.299 | + | | 1.299 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 33: | Line 37: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 184 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 184 | ||
Latest revision as of 08:20, 22 October 2022
Description
Pale yellow powder that is used as a pH indicator. Phenolphthalein is a triphenylmethane salt whose three aromatic rings produce a red chromophore in basic and neutral solutions. The conjugation is disrupted in acidic solutions resulting in a clear solution. Thus phenolphthalein is an effective acid base indicator for titrations of mineral acids, organic acids and alkalis. It is prepared as a 1% solution in ethanol. In solutions with a pH below 8.5, phenolphthalein is colorless and solutions above pH 9 are red.
Synonyms and Related Terms
3,3-bis(p-hydroxyphenyl)phthalide; 3,3-bis(4-hydroxyphenyl)-1(3H)-isobenzofuranore
Risks
- Toxic by ingestion, even small amounts will cause illness.
- Suspected carcinogen.
- Contact may cause irritation.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in ethanol, ether and alkalis. Insoluble in water.
Solution: Dissolve 1 g phenolphthalein in 50 ml ethanol and add 50 ml water.
| Composition | (C6H4OH)2C2O2C6H4 |
|---|---|
| CAS | 77-09-8 |
| Melting Point | 258-262 C |
| Density | 1.299 g/ml |
| Molecular Weight | mol. wt. = 318.33 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 184
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- S.R.Trotman, E.R. Trotman, Textile Analysis, J.B. Lippincott Company, Philadelphia, 1932
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: Solution: Dissolve 1 g phenolphthalein in 50 ml alcohol and add 50 ml water.