Difference between revisions of "Platina yellow"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 9: | Line 9: | ||
[[[SliderGallery rightalign|platina yellow.jpg~Chemical structure]]] | [[[SliderGallery rightalign|platina yellow.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Toxic by ingestion and inhalation. | ||
| + | * Contact may cause irritation or allergic reactions. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/01217.htm MSDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Slightly soluble in water. Insoluble in ethanol. | Slightly soluble in water. Insoluble in ethanol. | ||
| Line 22: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 250 (dec) | + | | 250 C (dec) |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3.499 | + | | 3.499 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 40: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 |
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.827 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.827 | ||
Latest revision as of 09:23, 26 July 2022
Description
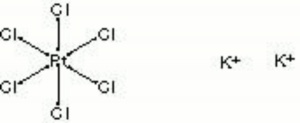
A yellow crystalline powder of potassium chloroplatinate. Platina yellow was sold for a short time in the late 18th and early 19th century as an artists pigment. It was initially called lemon yellow. Potassium chloroplatinate is now used in photography.
Synonyms and Related Terms
lemon yellow; potassium chloroplatinate; platinum (IV) potassium chloride; potassium platinichloride
Risks
- Toxic by ingestion and inhalation.
- Contact may cause irritation or allergic reactions.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Slightly soluble in water. Insoluble in ethanol.
| Composition | K2PtCl6 |
|---|---|
| CAS | 16921-30-5 |
| Melting Point | 250 C (dec) |
| Density | 3.499 g/ml |
| Molecular Weight | mol. wt. = 485.9946 |
| Refractive Index | 1.827 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.827