Difference between revisions of "Potassium bitartrate"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | White crystals or powder obtained from wine lees by extraction with water. Potassium bitartrate is used in baking powder, medicine, and as a mordant for dyeing textiles. It is used in combination with other mordants ([ | + | White crystals or powder obtained from wine lees by extraction with water. Potassium bitartrate is used in baking powder, medicine, and as a mordant for dyeing textiles. It is used in combination with other mordants ([[alum|alum]], [[tin|tin]]) to help maintain the softness of [[wool|wool]] fibers. It was also used for the galvanic tinning of metals and as a reducing agent in glass making. In the 16th and 17th century, impure potassium bitartrate, called [[argol|argol]], was used as a solder flux in combination with salt and alum. Mixed with water or [[vinegar|vinegar]], it has been used as a cleanser for encrusted metal objects, such as coins and kitchenware. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 11:30, 10 May 2016
Description
White crystals or powder obtained from wine lees by extraction with water. Potassium bitartrate is used in baking powder, medicine, and as a mordant for dyeing textiles. It is used in combination with other mordants (Alum, Tin) to help maintain the softness of Wool fibers. It was also used for the galvanic tinning of metals and as a reducing agent in glass making. In the 16th and 17th century, impure potassium bitartrate, called Argol, was used as a solder flux in combination with salt and alum. Mixed with water or Vinegar, it has been used as a cleanser for encrusted metal objects, such as coins and kitchenware.
Synonyms and Related Terms
cream of tartar; potassium acid tartrate; potassium hydrogen tartrate; cremor tartari; faecula; faecla; argol; lees; beeswing; Weinstein (Deut.); crème de tartre (Fr.)
Other Properties
Soluble in hot water, dilute acids, alkaline solutions.
Burn test gives purple flame
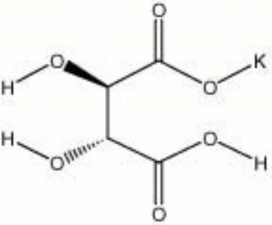
| Composition | KHC4H4O6 |
|---|---|
| CAS | 868-14-4 |
| Density | 1.984 |
| Molecular Weight | mol. wt. = 188.18 |
Hazards and Safety
Mallinckrodt Baker: MSDS
Sources Checked for Data in Record
- R. Mayer, The Artist's Handbook of Materials and Techniques, Viking Press, New York, 1981
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 68
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Palmy Weigle, Ancient Dyes for Modern Weavers, Watson-Guptill Publications, New York, 1974
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- John and Margaret Cannon, Dye Plants and Dyeing, Herbert Press, London, 1994
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7776
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Cream_of_tartar (Accessed Mar. 1, 2006)