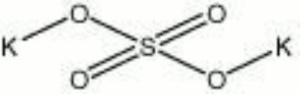
Potassium sulfate
Jump to navigation
Jump to search
Description
Colorless crystals or white powder. Potassium sulfate is used in the manufacture of gypsum cements, fertilizers, alums, and potassium glass. In a closed environment, a saturated solution of potassium sulfate will form an equilibrium at a relative humidity of about 97% (20C).
Synonyms and Related Terms
potassium sulphate (Br.); sulfate of potash; sulfate of potass; sal polychrest;
Risks
- Toxic in large amounts by ingestion.
- ThermoFisher: SDS
Physical and Chemical Properties
- Soluble in water (pH about 7) and glycerol. Insoluble in ethanol.
- Deliquescent point at 20C is 97.2 % RH (see saturated salt solutions)
| Composition | K2SO4 |
|---|---|
| CAS | 7778-80-5 |
| Melting Point | 1067-1072 C |
| Density | 2.66 g/ml |
| Molecular Weight | mol. wt. = 174.26 |
| Boiling Point | 1689 C |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 33
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7845