Difference between revisions of "Prussian blue"
| (12 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:1983.220-SC28842.jpg|thumb|]] | + | [[File:1983.220-SC28842.jpg|thumb|Pisarro<br> MFA#1983.220]] |
| + | [[File:GreatwaveMFA1117652.jpg|thumb|Great Wave<br>MFA 11.17652]] | ||
== Description == | == Description == | ||
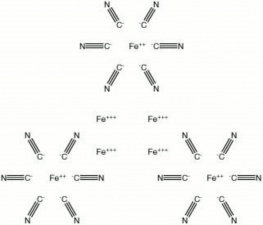
| − | A vivid, lightfast blue pigment composed of synthetically produced [[ferric%20 ferrocyanide|ferric ferrocyanide]]. Prussian blue was developed in Berlin in 1704 by Diesbach. It is made by adding ferric chloride to a boiling solution of hexacyano ferrate. This forms a white intermediate, called [[Berlin%20| | + | A vivid, lightfast blue pigment composed of synthetically produced [[ferric%20 ferrocyanide|ferric ferrocyanide]]. Prussian blue was developed in Berlin in 1704 by Diesbach. It is made by adding ferric chloride to a boiling solution of hexacyano ferrate. This forms a white intermediate, called [[Berlin%20 white|Berlin white]], that is oxidized in air to produce Prussian blue. Prussian blue has deep blue, finely divided particles that are transparent in watercolors. It has high tinting strength and is stable to light, although it turns brown in the presence of alkalis or heat. Prussian blue is used as a pigment in watercolor and oil paints as well as printing inks. It was also used as a colorant is [[cyanotype|cyanotype]]s, [[blueprint%20paper|blueprint paper]], [[laundry%20 blue|laundry blue]], [[linoleum|linoleum]], [[leather|leather]], [[plastic|plastics]], [[paper|paper]], and cosmetics. Prior to 1861, Prussian blue was used as a textile dye for [[silk|silk]], [[cotton|cotton]], and [[wool|wool]] where it was mordanted with [[ferric%20oxide|ferric oxide]]. For precision machining of parts, an oily film of Prussian blue pigment (engineer's blue) was placed on the fittings, then the parts were rubbed together; the removal of the pigment indicated the position of high spots or rubbing between the two surfaces. |
| − | |||
[[File:448 Prussian blue.jpg|thumb|Prussian blue]] | [[File:448 Prussian blue.jpg|thumb|Prussian blue]] | ||
| − | + | [[File:Prussianblue C100x.jpg|thumb|Prussian blue at 100x (visible light left; UV light right)]] | |
| + | [[File:38_Prussian_blue_500X.jpg|thumb|Prussian blue at 500x]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
ferric ferrocyanide; Pigment Blue 27; CI 77510; Preussisch Blau (Deut.); Berliner Blau (Deut.); bleu de Prusse (Fr,); Blu di Prussia (It.); blu di Parigi (It.); azzurro di Berlin (It.); azul de Prusia (Esp.); konjo (Jap.); bero ai (Jap.); yang lan (Chin.); mple ths Prossias (Gr.); Pruisisch blauw (Ned.); iron blue; azul da Prússia (Port.); ferrocyanide blue; Turnbull's blue; Paris blue; Milori blue; Chinese blue; bronze blue; Berlin blue; American blue; Antwerp blue; steel blue; mineral blue; Hamburg blue; toning blue; gas blue; new blue; Erlanger blue; celestial blue; lacquer blue; soluble blue; oriental blue; Persian blue; potash blue; paste blue; laundry blue; engineer's blue | ferric ferrocyanide; Pigment Blue 27; CI 77510; Preussisch Blau (Deut.); Berliner Blau (Deut.); bleu de Prusse (Fr,); Blu di Prussia (It.); blu di Parigi (It.); azzurro di Berlin (It.); azul de Prusia (Esp.); konjo (Jap.); bero ai (Jap.); yang lan (Chin.); mple ths Prossias (Gr.); Pruisisch blauw (Ned.); iron blue; azul da Prússia (Port.); ferrocyanide blue; Turnbull's blue; Paris blue; Milori blue; Chinese blue; bronze blue; Berlin blue; American blue; Antwerp blue; steel blue; mineral blue; Hamburg blue; toning blue; gas blue; new blue; Erlanger blue; celestial blue; lacquer blue; soluble blue; oriental blue; Persian blue; potash blue; paste blue; laundry blue; engineer's blue | ||
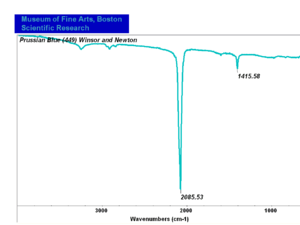
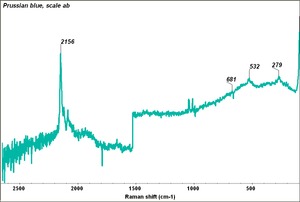
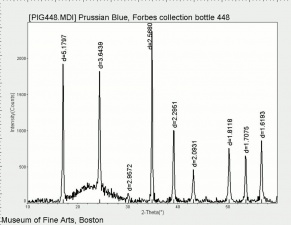
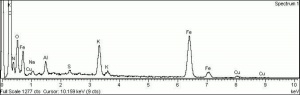
| − | [[[SliderGallery rightalign| | + | [[[SliderGallery rightalign|Prussian Blue (448).PNG~FTIR (MFA)|Prussian blue, scale ab.TIF~Raman (MFA)|PIG448.jpg~XRD|f448sem.jpg~SEM|f448edsbw.jpg~EDS|Prussian_Blue_FORS.JPG~FORS|prussian blue.jpg~Chemical structure]]] |
| + | == Risks == | ||
| − | == | + | * Low toxicity and nonmutagenic. |
| + | * Can emit highly toxic hydrogen cyanide fumes if exposed to acid, high heat or strong ultraviolet radiation. | ||
| + | * Ash contains ferric oxide. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/62402.htm MSDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in acids, decomposes in alkali. | Soluble in acids, decomposes in alkali. | ||
| Line 27: | Line 33: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.83 | + | | 1.83 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 35: | Line 41: | ||
| 1.56 | | 1.56 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 50: | Line 46: | ||
[[media:download_file_497.pdf|Characteristics of Common Blue Pigments]] | [[media:download_file_497.pdf|Characteristics of Common Blue Pigments]] | ||
| − | + | == Resources and Citations == | |
| − | + | * B.Berrie, "Prussian Blue", ''Artists Pigments'', Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997. | |
| − | == | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, Vols. 1 and 2, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, Vols. 1 and 2, 2004 | ||
| Line 94: | Line 81: | ||
* Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 | * Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 | ||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
| − | * | + | * Pigments through the ages - http://webexhibits.org/pigments/indiv/technical/prussblue.html |
* Colour Index International online at www.colour-index.org | * Colour Index International online at www.colour-index.org | ||
Latest revision as of 11:17, 29 November 2022
Description
A vivid, lightfast blue pigment composed of synthetically produced Ferric ferrocyanide. Prussian blue was developed in Berlin in 1704 by Diesbach. It is made by adding ferric chloride to a boiling solution of hexacyano ferrate. This forms a white intermediate, called Berlin white, that is oxidized in air to produce Prussian blue. Prussian blue has deep blue, finely divided particles that are transparent in watercolors. It has high tinting strength and is stable to light, although it turns brown in the presence of alkalis or heat. Prussian blue is used as a pigment in watercolor and oil paints as well as printing inks. It was also used as a colorant is cyanotypes, Blueprint paper, Laundry blue, Linoleum, Leather, plastics, Paper, and cosmetics. Prior to 1861, Prussian blue was used as a textile dye for Silk, Cotton, and Wool where it was mordanted with Ferric oxide. For precision machining of parts, an oily film of Prussian blue pigment (engineer's blue) was placed on the fittings, then the parts were rubbed together; the removal of the pigment indicated the position of high spots or rubbing between the two surfaces.
Synonyms and Related Terms
ferric ferrocyanide; Pigment Blue 27; CI 77510; Preussisch Blau (Deut.); Berliner Blau (Deut.); bleu de Prusse (Fr,); Blu di Prussia (It.); blu di Parigi (It.); azzurro di Berlin (It.); azul de Prusia (Esp.); konjo (Jap.); bero ai (Jap.); yang lan (Chin.); mple ths Prossias (Gr.); Pruisisch blauw (Ned.); iron blue; azul da Prússia (Port.); ferrocyanide blue; Turnbull's blue; Paris blue; Milori blue; Chinese blue; bronze blue; Berlin blue; American blue; Antwerp blue; steel blue; mineral blue; Hamburg blue; toning blue; gas blue; new blue; Erlanger blue; celestial blue; lacquer blue; soluble blue; oriental blue; Persian blue; potash blue; paste blue; laundry blue; engineer's blue
Risks
- Low toxicity and nonmutagenic.
- Can emit highly toxic hydrogen cyanide fumes if exposed to acid, high heat or strong ultraviolet radiation.
- Ash contains ferric oxide.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in acids, decomposes in alkali.
Cubic crystal system; isotropic. Very fine particles (0.01 - 0.2 micrometers) appear black under Chelsea filter.
| Composition | Fe4[Fe(CN)6]3 |
|---|---|
| CAS | 14038-43-8 |
| Density | 1.83 g/ml |
| Molecular Weight | mol. wt. = 859.25 |
| Refractive Index | 1.56 |
Comparisons
Characteristics of Common Blue Pigments
Resources and Citations
- B.Berrie, "Prussian Blue", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997.
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, Vols. 1 and 2, 2004
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: 'Pigment'
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- R.Feller, M.Curran, C.Bailie, 'Identification of Traditional Organic Colorants Employed in Japanese Prints and Determination of their Rates of Fading', Japanese Woodblock Prints, Allen Memorial Art Museum, Oberlin College, Oberlin, 1984
- A.Scharff, 'Synthetic dyestuffs for textiles and their fastness to washing', ICOM-CC Preprints Lyon, Getty Conservation Institute, Los Angeles, 1999
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 611
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4064
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Pigments through the ages - http://webexhibits.org/pigments/indiv/technical/prussblue.html
- Colour Index International online at www.colour-index.org