Difference between revisions of "Salicylic acid"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 44: | Line 44: | ||
== Authority == | == Authority == | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 872 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8484 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8484 | ||
| Line 56: | Line 56: | ||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
Revision as of 07:24, 24 July 2013
Description
A white crystalline powder. Salicylic acid occurs naturally in wintergreen leaves and the bark of sweet birch. It is prepared synthetically for used in the manufacture of aspirin and dyestuffs. Salicylic acid is also used as a preservative and fungicide in pastes.
Synonyms and Related Terms
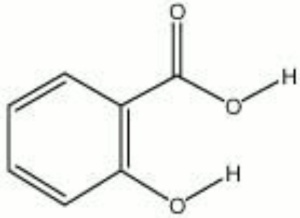
2-hydroxybenzoic acid; Keralyt; Occlusal; Verrugon; o-hydroxybenzoic acid
Other Properties
Soluble in hot water, ethanol, acetone, ether.
The fluorescence of salicylic acid changes with pH. It is colorless below pH=2.5 and blue fluorescent above 3.5.
| Composition | C6H4(OH)(COOH) |
|---|---|
| CAS | 69-72-7 |
| Melting Point | 157-161 |
| Density | 1.443 |
| Molecular Weight | mol. wt. = 138.1 |
| Boiling Point | 211 |
Hazards and Safety
Combustible. Toxic by ingestion of large amounts.
LINK: International Chemical Safety Card
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 872
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8484
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: The fluorescence of salicylic acid changes with pH. It is colorless below pH=2.5 and blue fluorescent above 3.5.
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998