Difference between revisions of "Steatite"
| (2 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
[[File:SC78821.jpg|thumb|Near Eastern bowl<br>MFA# 63.276]] | [[File:SC78821.jpg|thumb|Near Eastern bowl<br>MFA# 63.276]] | ||
A very soft rock composed primarily of the mineral [[talc|talc]]. Steatite, commonly called soapstone, is composed of hydrated magnesium silicate. It is easily cut and has been used for carvings since ancient times. Steatite is usually a white, grayish green, brown or in rare cases, red or black. The stones were used for bowls, boxes, and small objects such as figurines, beads, seals, amulets, and scarabs. Native steatite is so soft it can be scratched with a fingernail, but baking results in dehydration and hardening of the stone. Some ancient steatite carvings were glazed then fired which produced a mineral (enstatite) hard enough to scratch glass. Currently, soapstone is used for laboratory countertops and fireplace facings. | A very soft rock composed primarily of the mineral [[talc|talc]]. Steatite, commonly called soapstone, is composed of hydrated magnesium silicate. It is easily cut and has been used for carvings since ancient times. Steatite is usually a white, grayish green, brown or in rare cases, red or black. The stones were used for bowls, boxes, and small objects such as figurines, beads, seals, amulets, and scarabs. Native steatite is so soft it can be scratched with a fingernail, but baking results in dehydration and hardening of the stone. Some ancient steatite carvings were glazed then fired which produced a mineral (enstatite) hard enough to scratch glass. Currently, soapstone is used for laboratory countertops and fireplace facings. | ||
| − | + | [[File:2000.1293-SC116389.jpg|thumb|Soapstone sculpture<br>MFA# 2001.218]] | |
| − | [[File:2000.1293-SC116389.jpg|thumb|]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
soapstone; talc; enstatite (after firing); huashi (Chin.); Steatit (Deut.); esteatita (Esp.); stéatite (Fr.); pierre savon (Fr.); esteatito (Port.); pedra-sabão (Port.); French chalk; Spanish chalk; lard stone; pot-stone; pot stone | soapstone; talc; enstatite (after firing); huashi (Chin.); Steatit (Deut.); esteatita (Esp.); stéatite (Fr.); pierre savon (Fr.); esteatito (Port.); pedra-sabão (Port.); French chalk; Spanish chalk; lard stone; pot-stone; pot stone | ||
| − | |||
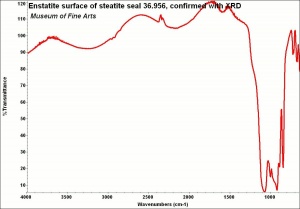
[[[SliderGallery rightalign|Enstatite.jpg~FTIR]]] | [[[SliderGallery rightalign|Enstatite.jpg~FTIR]]] | ||
| + | == Physical and Chemical Properties == | ||
| + | * Insoluble in water, cold acids or alkalis | ||
| + | * Ground particles can be very small (2.0 micrometers). | ||
| + | * Talc occurs naturally in foliated to fibrous masses | ||
| + | * Perfect cleavage in one direction producing thin laminar particles | ||
| + | * Fracture = uneven | ||
| + | * Luster = pearly, greasy | ||
| + | * Streak = white to pearl black | ||
| + | * Fluorescence = inert to weak pink or yellowish in LW; inert to pale orange in SW | ||
{| class="wikitable" | {| class="wikitable" | ||
| + | |- | ||
| + | ! scope="row"| Composition | ||
| + | | Mg3Si4O10(OH)2 | ||
| + | |- | ||
| + | ! scope="row"| CAS | ||
| + | | 14807-96-6 | ||
|- | |- | ||
! scope="row"| Mohs Hardness | ! scope="row"| Mohs Hardness | ||
| − | | 1 | + | | 1.0 |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| Line 21: | Line 34: | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| 1.539; 1.589; 1.589 | | 1.539; 1.589; 1.589 | ||
| + | |- | ||
| + | ! scope="row"| Birefringence | ||
| + | | 0.050 - 0.051 | ||
|} | |} | ||
| Line 26: | Line 42: | ||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density=2.77; ref. index=1.539; 1.589; 1.589 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density=2.77; ref. index=1.539; 1.589; 1.589 | ||
| − | + | * Gem Identification Lab Manual, Gemological Institute of America, 2016. | |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 733, 793 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 733, 793 | ||
| − | |||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | |||
* Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | * Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | ||
| − | |||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| − | |||
* A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 | * A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 | ||
| − | |||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | |||
* Marble Institute: http://www.marble-institute.com | * Marble Institute: http://www.marble-institute.com | ||
| − | |||
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=2.6-2.8 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=2.6-2.8 | ||
| − | |||
* Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | ||
Latest revision as of 15:03, 28 December 2022
Description
A very soft rock composed primarily of the mineral Talc. Steatite, commonly called soapstone, is composed of hydrated magnesium silicate. It is easily cut and has been used for carvings since ancient times. Steatite is usually a white, grayish green, brown or in rare cases, red or black. The stones were used for bowls, boxes, and small objects such as figurines, beads, seals, amulets, and scarabs. Native steatite is so soft it can be scratched with a fingernail, but baking results in dehydration and hardening of the stone. Some ancient steatite carvings were glazed then fired which produced a mineral (enstatite) hard enough to scratch glass. Currently, soapstone is used for laboratory countertops and fireplace facings.
Synonyms and Related Terms
soapstone; talc; enstatite (after firing); huashi (Chin.); Steatit (Deut.); esteatita (Esp.); stéatite (Fr.); pierre savon (Fr.); esteatito (Port.); pedra-sabão (Port.); French chalk; Spanish chalk; lard stone; pot-stone; pot stone
Physical and Chemical Properties
- Insoluble in water, cold acids or alkalis
- Ground particles can be very small (2.0 micrometers).
- Talc occurs naturally in foliated to fibrous masses
- Perfect cleavage in one direction producing thin laminar particles
- Fracture = uneven
- Luster = pearly, greasy
- Streak = white to pearl black
- Fluorescence = inert to weak pink or yellowish in LW; inert to pale orange in SW
| Composition | Mg3Si4O10(OH)2 |
|---|---|
| CAS | 14807-96-6 |
| Mohs Hardness | 1.0 |
| Density | 2.7-2.8 g/ml |
| Refractive Index | 1.539; 1.589; 1.589 |
| Birefringence | 0.050 - 0.051 |
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density=2.77; ref. index=1.539; 1.589; 1.589
- Gem Identification Lab Manual, Gemological Institute of America, 2016.
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 733, 793
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Marble Institute: http://www.marble-institute.com
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=2.6-2.8
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985