Aluminum oxide
Description
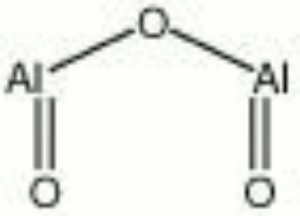
A white, hard, insoluble powder. Aluminum oxide, or alumina, naturally occurs in some feldspars, Alumina trihydrate (Al(H2O)3), Corundum (Al2O3), gibbsite (Al(OH)3), Bauxite, Ruby, and Sapphire. It was first extract commercially from bauxite in 1888 using the Bayer process. Aluminum oxide is extremely hard and is used as an Abrasive both in its natural (corundum, Emery) and synthetic (Alundum) forms. Synthetic alumina is prepared primarily in three forms: activated alumina, smelter-grade alumina, and calcined alumina. The porous, granular activated alumina aggressively absorbs liquid water and vater vapor. The fine-grain calcined alumina is a dense impermeable ceramic material used for abrasives, refractories, electrical insulation, high temperature crucibles and dental restoration. It is also used as a filler for paints, glass, and ceramics. When added to glaze mixtures, aluminum oxide increases viscosity during firing, prevents devitrification during cooling and adds durability to the final surface.
Synonyms and Related Terms
aluminium oxide (IUPAC); alumina; corundum; emery; aluminiumoxyd (Dan.); Aluminiumoxid (Deut.); alumine (Fr.); oxyde d'aluminium (Fr.); aluminiumoxide (Ned.); Aluminiumoksid (Nor.); trójtlenek glinu (Pol.); óxido de alumínio (Port.); Alundum; Aloxite; Bauxilite; White Bauxilite; Lucalox [GE]
Risks
Inhalation of dust may cause irritation. Fire retardant.
Fisher Scientific: SDS
Physical and Chemical Properties
Soluble in mineral acids and strong alkali. Insoluble in water.
| Composition | Al2O3 |
|---|---|
| CAS | 1344-28-1 |
| Mohs Hardness | 8.0 - 9.0 |
| Melting Point | 2030-2050 |
| Density | 2.8-4.0 |
| Molecular Weight | mol. wt. = 101.9 |
| Refractive Index | 1.665-1.680, 1.63-1.65 |
Comparisons
Properties of Common Abrasives
Properties of Common Gemstones
Properties of Natural and Simulated Diamonds
Additional Images
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 34
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- R.M.Organ, Design for Scientific Conservation of Antiquities, Smithsonian Institution, Washington DC, 1968
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Robert Fournier, Illustrated Dictionary of Practical Pottery, Chilton Book Company, Radnor, PA, 1992
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 369
- Encyclopedia Britannica, http://www.britannica.com Comment: "Alumina." Accessed 02 Dec, 2003 .
- Wikipedia: http://en.wikipedia.org/wiki/Aluminium_oxide (Accessed Mar. 15, 2006) for non-English terms
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.665-1.680, 1.63-1.65
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998