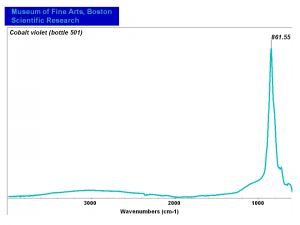
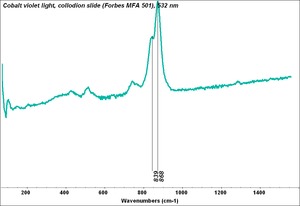
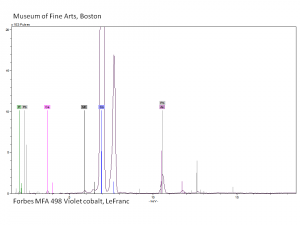
Cobaltous arsenate
Jump to navigation
Jump to search
Description
A pale to medium violet pigment. Cobaltous arsenate, or light cobalt violet, occurs in nature as cobalt bloom or erythrite. Once it was synthetically produced in 1880, it became an important permanent, violet pigment. Cobaltous arsenate is now rarely used because of its toxicity. It has been replaced by the use of cobaltous phosphate and cobaltous ammonium phosphate. Cobaltous arsenate was used as a colorant in paints, glass, glazes, and enamels.
Synonyms and Related Terms
cobalt violet light; Pigment Violet 14; CI 77350; erythrite (mineral); arseniato de cobalto (Esp.); arsenate de cobalt (Fr.); arseniato di cobalto (It.); arsenato de cobalto (Port.); cobalt arsenate; cobalt bloom;
Risks
Highly toxic by ingestion, inhalation, and skin contact.
Physical and Chemical Properties
- Soluble in dilute mineral acids and ammonium hydroxide. Insoluble in water.
- Prismatic or euhedral crystals; perfect cleavage parallel to long axes
- Weakly pleochroic
- High birefringence
| Composition | Co3(AsO4)2 - 8H2O |
|---|---|
| Density | 3.06 g/ml |
| Molecular Weight | mol. wt. = 454.64 |
| Refractive Index | 1.626-1.701 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2495
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: prepared first in 1880