Difference between revisions of "Barium nitrate"
Jump to navigation
Jump to search
(username removed) |
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
||
| Line 33: | Line 33: | ||
Mallinckrodt Baker: [http://www.jtbaker.com/msds/englishhtml/b0432.htm MSDS] | Mallinckrodt Baker: [http://www.jtbaker.com/msds/englishhtml/b0432.htm MSDS] | ||
| − | == | + | == Sources Checked for Data in Record == |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 Comment: melting point=575C | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 Comment: melting point=575C | ||
Revision as of 14:00, 29 April 2016
Description
White crystals or fused mass that is a strong oxidizing agent. . Barium nitrate is primarily used to produce green fireworks and green signal lights. It is also used in the manufacture of glass and ceramic glazes as well as for a rodenticide.
Other Properties
Soluble in water. Insoluble in ethanol.
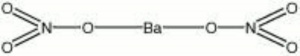
| Composition | Ba(NO3)2 |
|---|---|
| CAS | 10022-31-8 |
| Melting Point | 575 |
| Density | 3.244 |
| Molecular Weight | mol. wt. = 261.34 |
Hazards and Safety
Highly toxic by ingestion. Contact with skin and membranes may cause irritation.
Mallinckrodt Baker: MSDS
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993 Comment: melting point=575C
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1012
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Barium_nitrate (Accessed Jan. 6, 2006) has melting point = 595C