Difference between revisions of "Lindane"
Jump to navigation
Jump to search
(username removed) |
|||
| (4 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
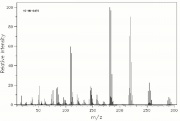
| − | [[File: | + | [[File:lindanems.jpg|thumb|Mass spectrum of lindane]] |
== Description == | == Description == | ||
| − | The legal name for the chemical gamma benzene hexachloride. Lindane is a powerful [ | + | The legal name for the chemical gamma benzene hexachloride. Lindane is a powerful [[insecticide]] that is used as a fungicide, an indoor fumigant, and a contact poison. It is 100 times more toxic against roaches than [[DDT]] killing both insects and larvae. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 8: | Line 8: | ||
gamma benzene hexachloride; benzene hexachloride; BHC; Gammexane [ICI]; hexachlorocyclohexane; Insecticide 666; Tri-6; Viton; gamma hexachlor; Lindafor; Lindatox; Agrocide; Isotox; Esoderm; Aparasin; | gamma benzene hexachloride; benzene hexachloride; BHC; Gammexane [ICI]; hexachlorocyclohexane; Insecticide 666; Tri-6; Viton; gamma hexachlor; Lindafor; Lindatox; Agrocide; Isotox; Esoderm; Aparasin; | ||
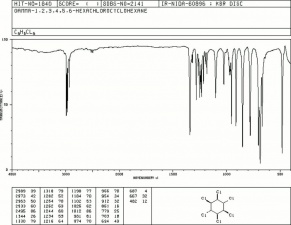
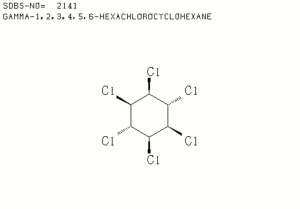
| − | [[[SliderGallery rightalign|lindanestruct.jpg~Chemical structure]]] | + | [[[SliderGallery rightalign|lindaneIR.jpg~FTIR|lindanestruct.jpg~Chemical structure]]] |
| − | == | + | == Risks == |
| + | |||
| + | * Toxic by ingestion, inhalation, and skin absorption. LD50 = 88-270 mg/kg | ||
| + | * Chem Service: [http://cdn.chemservice.com/product/msdsnew/External/English/N-12319%20English%20SDS%20US.pdf SDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in acetone, cyclohexane, naphtha, benzene, and ethanol. Insoluble in water. | Soluble in acetone, cyclohexane, naphtha, benzene, and ethanol. Insoluble in water. | ||
| Line 23: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 112.5 | + | | 112.5 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.87 | + | | 1.87 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 32: | Line 37: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 323 | + | | 323 C |
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * Thomas Gregory, ''The Condensed Chemical Dictionary'', Reinhold Publishing, New York, 3rd ed., 1942 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry # 5526 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry # 5526 | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 413 |
| − | * | + | * Lynda A. Zycherman, J.Richard Schrock, ''A Guide to Museum Pest Control'', FAIC and Association of Systematics Collections, Washington DC, 1988 |
| − | * | + | * Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 |
| − | * | + | * G.Caneva, M.P.Nugari, O.Salvadori, ''Biology in the Conservation of Works of Art'', ICCROM, Rome, 1991 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 12:41, 8 September 2022
Description
The legal name for the chemical gamma benzene hexachloride. Lindane is a powerful Insecticide that is used as a fungicide, an indoor fumigant, and a contact poison. It is 100 times more toxic against roaches than DDT killing both insects and larvae.
Synonyms and Related Terms
gamma benzene hexachloride; benzene hexachloride; BHC; Gammexane [ICI]; hexachlorocyclohexane; Insecticide 666; Tri-6; Viton; gamma hexachlor; Lindafor; Lindatox; Agrocide; Isotox; Esoderm; Aparasin;
Risks
- Toxic by ingestion, inhalation, and skin absorption. LD50 = 88-270 mg/kg
- Chem Service: SDS
Physical and Chemical Properties
Soluble in acetone, cyclohexane, naphtha, benzene, and ethanol. Insoluble in water.
| Composition | C6H6Cl6 |
|---|---|
| CAS | 58-89-9 |
| Melting Point | 112.5 C |
| Density | 1.87 g/ml |
| Molecular Weight | mol. wt.=290.8 |
| Boiling Point | 323 C |
Resources and Citations
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry # 5526
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 413
- Lynda A. Zycherman, J.Richard Schrock, A Guide to Museum Pest Control, FAIC and Association of Systematics Collections, Washington DC, 1988
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- G.Caneva, M.P.Nugari, O.Salvadori, Biology in the Conservation of Works of Art, ICCROM, Rome, 1991