Difference between revisions of "Witherite"
Jump to navigation
Jump to search
| Line 10: | Line 10: | ||
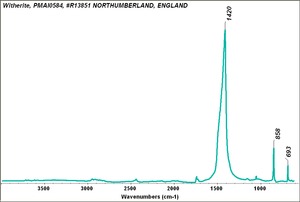
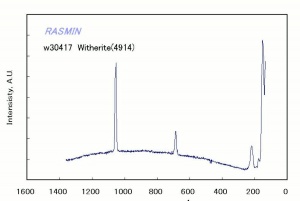
[[[SliderGallery rightalign|Witherite, PMA.TIF~FTIR (PMA)|witheriteRS.jpg~Raman]]] | [[[SliderGallery rightalign|Witherite, PMA.TIF~FTIR (PMA)|witheriteRS.jpg~Raman]]] | ||
| − | == | + | == Risks == |
| − | + | * Toxic by ingestion. | |
| + | * Skin contact may cause irritation. | ||
| − | + | ==Physical and Chemical Properties== | |
| − | Fracture = subconchoidal. Luster = veitreous to resinous. Streak = white. | + | * Soluble in acids. Insoluble in water. |
| − | + | * Orthorhombic crystals, usually short and prismatic. | |
| − | Flat tablets that are colorless under plane-polarized light; high brefringence with complete extinction; interference colors are often seen. | + | * Cleavage is good in one direction. |
| − | + | * Fracture = subconchoidal. | |
| − | Fluoresces a light blue color in both long- and short-wave UV and is phosphorescent under short-wave UV light | + | * Luster = veitreous to resinous. |
| + | * Streak = white. | ||
| + | * Flat tablets that are colorless under plane-polarized light; high brefringence with complete extinction; interference colors are often seen. | ||
| + | * Fluoresces a light blue color in both long- and short-wave UV and is phosphorescent under short-wave UV light | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 28: | Line 32: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 4.3 | + | | 4.3 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| 1.529-1.677 | | 1.529-1.677 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 46: | Line 42: | ||
[[media:download_file_538.pdf|Characteristics of Common White Pigments]] | [[media:download_file_538.pdf|Characteristics of Common White Pigments]] | ||
| + | ==Resources and Citations== | ||
| − | + | * Mineralogy Database: [http://www.webmineral.com/data/Witherite.shtml Witherite] | |
| − | |||
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Vol. 2, page 267. Refractive index: alpha=1.529, beta=1.676, gamma=1.677. Microscopically similar to aragontie | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Vol. 2, page 267. Refractive index: alpha=1.529, beta=1.676, gamma=1.677. Microscopically similar to aragontie | ||
| Line 58: | Line 54: | ||
* C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Witherite (Accessed Sept. 20, 2005) |
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
Revision as of 11:41, 27 June 2022
Description
A naturally occurring mineral composed of Barium carbonate. Witherite was named for william Withering an 18th century English naturalist. It is usually found in hydrothermal veins associated with Barite. Witherite is mined in Germany, England (Hexham, Northumberland, Cumberland, Durham), Scotland, Canada (Ontario) and the U.S. (Illinois, California, Arizona). Witherite is used in case-hardening Steel and in refining Sugar. It is also used as a pigment in the manufacture of paints, glazes, and synthetic Marble.
Synonyms and Related Terms
barium carbonate; Pigment White 10; CI 77099
Risks
- Toxic by ingestion.
- Skin contact may cause irritation.
Physical and Chemical Properties
- Soluble in acids. Insoluble in water.
- Orthorhombic crystals, usually short and prismatic.
- Cleavage is good in one direction.
- Fracture = subconchoidal.
- Luster = veitreous to resinous.
- Streak = white.
- Flat tablets that are colorless under plane-polarized light; high brefringence with complete extinction; interference colors are often seen.
- Fluoresces a light blue color in both long- and short-wave UV and is phosphorescent under short-wave UV light
| Mohs Hardness | 3.0 - 3.5 |
|---|---|
| Density | 4.3 g/ml |
| Refractive Index | 1.529-1.677 |
Comparisons
Characteristics of Common White Pigments
Resources and Citations
- Mineralogy Database: Witherite
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Vol. 2, page 267. Refractive index: alpha=1.529, beta=1.676, gamma=1.677. Microscopically similar to aragontie
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 84
- Encyclopedia Britannica, http://www.britannica.com Comment: "witherite" Encyclopædia Britannica [Accessed December 4, 2001
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Wikipedia: http://en.wikipedia.org/wiki/Witherite (Accessed Sept. 20, 2005)
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982