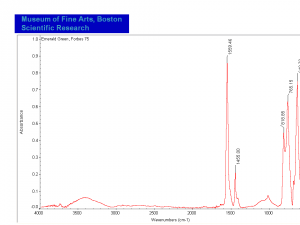
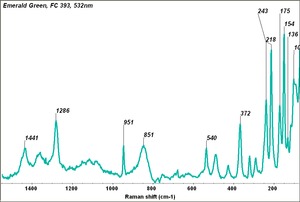
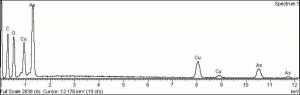
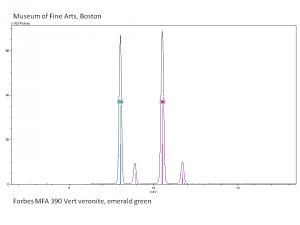
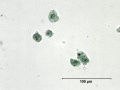
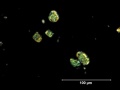
Emerald green
Description
A clear, bright green, synthetic pigment composed of Copper acetoarsenite. Emerald green was discovered about 1800 and first commercially manufactured in Schweinfurt Germany in 1814. It is extremely poisonous. Emerald green is lightfast but is decomposed by acids and warm alkalis and darkens in the presence of sulfur. Emerald green was used in the 19th century for oil paints, coach paints, watercolors, pastels, wax crayons and pencils. In the early 20th century, it was also used as a colorant in wallpapers, fabrics, linoleum, and toys. Marketed as Paris green, it was used as an Insecticide, fungicide, and rat poison. Emerald green is no longer used as a pigment due to its toxicity. The name emerald green is not standardized and has also been used for chrome oxide greens and synthetic dyes. Another green copper-arsenic pigment is Copper arsenite (Scheele's green).
Synonyms and Related Terms
copper acetoarsenite; Pigment Green 21; CI 77410; vert cendre (Fr.); vert Véronèse (Fr.); verde ceniza (Esp.); verde esmeralda (Esp.); Deckgrun (Deut.); Schweinfurter Grünn (Deut.); prasino Emerald (Gr.); verde di Schweinfurt (It.); verde di Parigi (It.), schweinfurter Groen (Ned.); verde esmeralda (Port.); cupric acetoarsenite; copper acetate arsenite; king's green; Paris green; Schweinfurt green; mineral green; imperial green; Mitis green; parrot green; Vienna green; new green; patent green; Emperor green; Kaiser green; meadow green; English green; Imperial green
Risks
- Extremely toxic; human carcinogen.
- Skin contact may cause irritation, ulceration and cancer.
- Ingestion may be fatal.
- Gamblin Colors: SDS
Physical and Chemical Properties
- Soluble in mineral acids. Insoluble in water.
- Decomposes in alkalis.
- Darkens in the presence of sulfur or lead compounds.
- Small rounded grains with radial structure and dark spot in the middle. High birefringence.
| Composition | Cu(C2H3O2)2.3Cu(AsO2)2 |
|---|---|
| Density | 3.27 g/ml |
| Molecular Weight | mol. wt. = 1013.8 |
| Refractive Index | 1.71(alpha); 1.78(beta) |
Additional Images
Resources and Citations
- I.Fiedler, M Bayard, "Emerald Green and Scheele's Green", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997.
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2692
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 3.27 and ref.index = 1.71; 1.78
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: 'Pigment'
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Monona Rossol, The Artist's Complete Health and Safety Guide, Allworth Press, New York, 1994
- R. Newman, E. Farrell, 'House Paint Pigments', Paint in America , R. Moss ed., Preservation Press, New York City, 1994
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- David Bomford, Jo Kirby, John Leighton, Ashok Roy, Art in the Making:Impressionism, National Gallery, London, 1990
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Pigments Through the Ages: http://webexhibits.org/pigments/indiv/overview/emerald.html ref index: alpha = 1.71-2, beta = 1.77-8