Teflon
Description
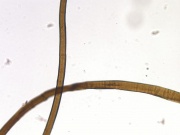
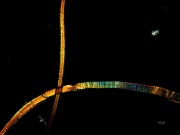
[DuPont] A registered trademark for a solid white polymer that is resistant to almost all chemicals. Polytetrafluoroethylene was accidentally discovered in 1938 by Roy Plunkett at DuPont during an attempt to synthesize tetrafluoroethylene. The new polymer was found to be resistant to heat, chemical, light, and adhesion and subsequently marketed as Teflon® by DuPont in 1943. Teflon® is a soft, opaque material that is unaffected by acids, alkalis, and organic solvents. It is widely used for containers in chemical plants, for rockets, bearings, gaskets, and for frying pan coatings. Teflon® is used for stain-resistant, water-repellent coatings on textiles. It can also be prepared as ribbonlike fibers, which are woven, knitted, felted or braided. Additionally, GORE-TEX® is prepared from a microporous Teflon® film laminated on a polyester fabric.
See also fluorinated ethylene propylene.
Synonyms and Related Terms
PTFE; polytetrafluoroethylene; Polytetrafluorethylen (Deut.); politetrafluoretileno (Esp.); polyttrafluorthylne (Fr.); Teflon (Port.)
Examples: GORE-TEX [W.R.Gore]; Teflon TFE [DuPont]; Rulon [Saint-Gobain]; Algoflon [Solvay]; Fluorosint [Quadrant];
Personal Risks
Heated to temperatures consistent with everyday cooking, Teflon emits minute particles that can lodge deeply in the lungs. These particles are deadly to pet birds and can cause a disease known as polymer fume fever, a severe flu-like condition, in humans. Does not burn in flame but evaporates above 215C and evolves HF.
Environmental Risks
Perfluorooctanoic acid (PFOA), also known as C8, is aman-made chemical used in the process of making Teflon that the potential to be a health concern because it can stay in the environment and in the human body for long periods of time. PFOA and some similar compounds can be found at low levels in some foods, drinking water, and in household dust. Although PFOA levels in drinking water are usually low, they can be higher in certain areas, such as near chemical plants, such as DuPont, that use or make PFOA compounds.
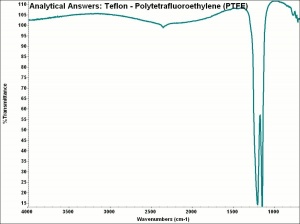
Physical and Chemical Properties
- Highly resistant to acids, alkalis and solvents
- Tenacity = 0.5-1.4 g/denier; Elongation = 15-32 %
- Moisture regain = 0%; 1 mil films: 0.1 g/m2d
- Melting Point = 300 (dec)
- Density = 2.1-2.3
Resources and Citations
- DuPont: Teflon Website
- Rachael Perkins Arenstein, Lisa Goldberg, and Eugenie Milroy, ‘Support and Rehousing for Collection Storage’ In ‘Preventive Conservation: Collection Storage’ Lisa Elkin and Christopher A. Norris (eds.), Society for the Preservation of Natural History Collections, New York. 2019.
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- Marjory L. Joseph, Introductory Textile Science, Holt, Rinehart and Winston, Fort Worth, TX, 1986
- Identification of Textile Materials, The Textile Institute, Manchester, England, 1985
- J.Gordon Cook, Handbook of Textile Fibres:II Man-made Fibres, Merrow Publishing Co. , Durham, England, p.509
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988