Difference between revisions of "Acetone"
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | An important colorless, volatile, liquid solvent with a sweet odor. Acetone is obtained as a byproduct of wood distillation or the fermentation of corn mash. It has been known since the 19th century where it was used as a solvent for paints, varnishes, and many of the newly developed plastics. Acetone was also used for dyeing [http://cameo.mfa.org/materials/fullrecord.asp?name=cotton cotton] with [http://cameo.mfa.org/materials/fullrecord.asp?name=aniline | + | An important colorless, volatile, liquid solvent with a sweet odor. Acetone is obtained as a byproduct of wood distillation or the fermentation of corn mash. It has been known since the 19th century where it was used as a solvent for paints, varnishes, and many of the newly developed plastics. Acetone was also used for dyeing [http://cameo.mfa.org/materials/fullrecord.asp?name=cotton cotton] with [http://cameo.mfa.org/materials/fullrecord.asp?name=aniline%20black aniline black] and dewaxing [http://cameo.mfa.org/materials/fullrecord.asp?name=oil oils]. It will dissolve many common materials such as plastic eyeglasses, nail polish, adhesive tape, pens, [http://cameo.mfa.org/materials/fullrecord.asp?name=viscose%20rayon rayon], plastic containers, [http://cameo.mfa.org/materials/fullrecord.asp?name=ink inks], [http://cameo.mfa.org/materials/fullrecord.asp?name=dye dyes], photographic film, [http://cameo.mfa.org/materials/fullrecord.asp?name=wax waxes], [http://cameo.mfa.org/materials/fullrecord.asp?name=shellac shellac], rubbers, and coatings. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 51: | Line 51: | ||
== Authority == | == Authority == | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p.9 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: ref. index=1.3591 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: ref. index=1.3591 | ||
| − | * | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 |
| − | * | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) |
| − | * | + | * Hoechst Celanese Corporation, ''Dictionary of Fiber & Textile Technology'' (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990 |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 |
| − | * | + | * Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
Revision as of 06:24, 24 July 2013
Description
An important colorless, volatile, liquid solvent with a sweet odor. Acetone is obtained as a byproduct of wood distillation or the fermentation of corn mash. It has been known since the 19th century where it was used as a solvent for paints, varnishes, and many of the newly developed plastics. Acetone was also used for dyeing cotton with aniline black and dewaxing oils. It will dissolve many common materials such as plastic eyeglasses, nail polish, adhesive tape, pens, rayon, plastic containers, inks, dyes, photographic film, waxes, shellac, rubbers, and coatings.
Synonyms and Related Terms
dimethylketone; dimethylketal; ketopropane; 2-propanone
Other Properties
Miscible with water, ethanol, dimethylformamide, chloroform, ether and most oils.
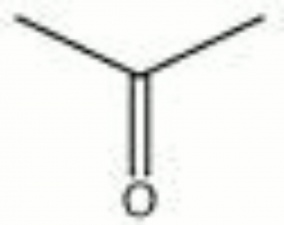
| Composition | CH3COCH3 |
|---|---|
| CAS | 67-64-1 |
| Melting Point | -94 |
| Density | 0.788 |
| Molecular Weight | mol. wt. = 58.08 |
| Refractive Index | 1.3591 |
| Boiling Point | 56.5 |
Hazards and Safety
Highly flammable with a low flash point (-18C). Overexposure can cause skin, eye, nose and throat irritation, headache and dizziness. Ingestion causes vomiting.
LINK: International Chemical Safety Card
Comparisons
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p.9
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: ref. index=1.3591
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.357