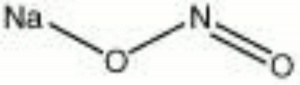Difference between revisions of "Sodium nitrite"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | White to pale yellow [http://cameo.mfa.org/materials/fullrecord.asp?name=hygroscopic hygroscopic] crystals. Sodium nitrite is used in dyeing [http://cameo.mfa.org/materials/fullrecord.asp?name=textile textiles] with [http://cameo.mfa.org/materials/fullrecord.asp?name=developed | + | White to pale yellow [http://cameo.mfa.org/materials/fullrecord.asp?name=hygroscopic hygroscopic] crystals. Sodium nitrite is used in dyeing [http://cameo.mfa.org/materials/fullrecord.asp?name=textile textiles] with [http://cameo.mfa.org/materials/fullrecord.asp?name=developed%20dye developed dyes]. It is also used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=fixative fixative] for color photographs. In a closed environment, a saturated solution of sodium nitrite will form an equilibrium at a relative humidity of about 65% (20C). |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 13: | Line 13: | ||
Soluble in water. Slightly soluble in ethanol. | Soluble in water. Slightly soluble in ethanol. | ||
| − | Deliquescent point at 20C is 65.3 % RH (see [http://cameo.mfa.org/materials/fullrecord.asp?name=saturated | + | Deliquescent point at 20C is 65.3 % RH (see [http://cameo.mfa.org/materials/fullrecord.asp?name=saturated%20salt%20solutions saturated salt solutions]) |
{| class="wikitable" | {| class="wikitable" | ||
| Line 41: | Line 41: | ||
== Authority == | == Authority == | ||
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8793 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8793 | ||
Revision as of 06:30, 24 July 2013
Description
White to pale yellow hygroscopic crystals. Sodium nitrite is used in dyeing textiles with developed dyes. It is also used as a fixative for color photographs. In a closed environment, a saturated solution of sodium nitrite will form an equilibrium at a relative humidity of about 65% (20C).
Synonyms and Related Terms
nitrous acid sodium salt; erinitrit; nitrite
Other Properties
Soluble in water. Slightly soluble in ethanol.
Deliquescent point at 20C is 65.3 % RH (see saturated salt solutions)
| Composition | NaNO2 |
|---|---|
| CAS | 7632-00-0 |
| Melting Point | 271 |
| Density | 2.157 |
| Molecular Weight | mol. wt. = 69.0 |
Hazards and Safety
Carcinogenic in test animals. Strong oxidizing agent. Fire risk in contact with oxidizing materials. Used as an antidote for cyanide poisoning.
LINK: International Chemical Safety Card
Authority
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8793