Difference between revisions of "Propylene oxide"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
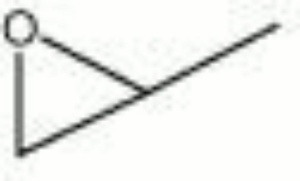
== Description == | == Description == | ||
| − | A colorless, flammable liquid with an ether-like odor. Propylene oxide is used to make [http://cameo.mfa.org/materials/fullrecord.asp?name=propylene | + | A colorless, flammable liquid with an ether-like odor. Propylene oxide is used to make [http://cameo.mfa.org/materials/fullrecord.asp?name=propylene%20glycol propylene glycol] and [http://cameo.mfa.org/materials/fullrecord.asp?name=polyurethane urethane] foams. It is also used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=fumigant fumigant], [http://cameo.mfa.org/materials/fullrecord.asp?name=biocide biocide], and [http://cameo.mfa.org/materials/fullrecord.asp?name=solvent solvent]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 42: | Line 42: | ||
== Authority == | == Authority == | ||
| − | * | + | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 8041 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 07:33, 24 July 2013
Description
A colorless, flammable liquid with an ether-like odor. Propylene oxide is used to make propylene glycol and urethane foams. It is also used as a fumigant, biocide, and solvent.
Synonyms and Related Terms
1,2-epoxypropane; methyloxirane; methyl ethylene oxide; propene oxide
Other Properties
Degrades to for propylene glycol in the presence of water.
| Composition | C3H6O |
|---|---|
| CAS | 75-56-9 |
| Melting Point | -112.13 |
| Density | 0.859 |
| Molecular Weight | mol. wt. = 58.08 |
| Boiling Point | 34.23 |
Hazards and Safety
Highly flammable. Strong oxidizer. Flash point - -35C Contact causes burns. Potential carcinogen.
LINK: International Chemical Safety Card
Authority
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 8041
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993