Difference between revisions of "Sodium bisulfite"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | White crystals or powder. Sodium bisulfite is used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=disinfectant disinfectant] and [http://cameo.mfa.org/materials/fullrecord.asp?name=bleaching | + | White crystals or powder. Sodium bisulfite is used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=disinfectant disinfectant] and [http://cameo.mfa.org/materials/fullrecord.asp?name=bleaching%20agent bleach], especially for wool and leather. It is also used as a bleach, digestive aid, and [http://cameo.mfa.org/materials/fullrecord.asp?name=antichlor%20agent antichlor] in papermaking vats. Sodium bisulfite is also used in photographic developing solutions and dye baths as a reducing agent. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 44: | Line 44: | ||
== Authority == | == Authority == | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 785 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 |
* ''The Dictionary of Paper'', American Paper Institute, New York, Fourth Edition, 1980 | * ''The Dictionary of Paper'', American Paper Institute, New York, Fourth Edition, 1980 | ||
Revision as of 06:34, 24 July 2013
Description
White crystals or powder. Sodium bisulfite is used as a disinfectant and bleach, especially for wool and leather. It is also used as a bleach, digestive aid, and antichlor in papermaking vats. Sodium bisulfite is also used in photographic developing solutions and dye baths as a reducing agent.
Synonyms and Related Terms
sodium acid sulfite; sodium hydrogen sulfite; sodium metabisulfite; sodium pyrosulfite
Other Properties
Soluble in water. Insoluble in ethanol.
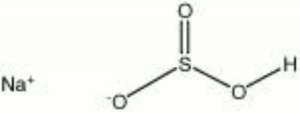
| Composition | NaHSO3 |
|---|---|
| CAS | 7631-90-5 |
| Melting Point | 150 |
| Density | 1.48 |
| Molecular Weight | mol. wt. = 104.06 |
| Refractive Index | 1.474, 1.526, 1.685 |
Hazards and Safety
Noncombustible. Contact causes irritation and burns. Toxic by ingestion.
Reacts with acids to evolve toxic sulfur dioxide fumes.
Mallinckrodt Baker: MSDS
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 785
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8731
- A Glossary of Paper Conservation Terms, Margaret Ellis (ed.), Conservation Center of the Institute of Fine Arts, New York City, 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index= 1.474, 1.526, 1.685