Difference between revisions of "Safranine"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 39: | Line 39: | ||
== Authority == | == Authority == | ||
| − | * | + | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 |
| − | * | + | * F. Crace-Calvert, ''Dyeing and Calico Printing'', Palmer & Howe, London, 1876 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
| − | * | + | * Colour Index International online at www.colour-index.org |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 06:44, 24 July 2013
Description
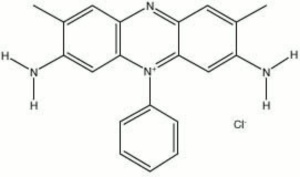
A family of red organic dyes derived from phenazine. Safranine dyes are used for textiles (wool, silk) and leather without a mordant. They are also used as biological stains for bacteria in a Gram's test.
- Safranine O = CI 50240; Basic Red 2
- Safranine T = CI 50240
Synonyms and Related Terms
Basic Red 2; safranina (Esp.); CI 50240; safranin; saffranine; phenosafranine
Other Properties
Soluble in water and alcohol.
When concentrated sulfuric acid is added to a safranine solution, it will change from red to violet to blue to green. Once green, water can be added to dilute the solution and the colors will change back in reverse order.
| Composition | C20H19N4Cl |
|---|---|
| CAS | 477-73-6 (safranine O) |
| Molecular Weight | mol. wt. = 350.85 |
Hazards and Safety
Contact causes irritation.
Mallinckrodt Baker: MSDS
Authority
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- F. Crace-Calvert, Dyeing and Calico Printing, Palmer & Howe, London, 1876
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- Colour Index International online at www.colour-index.org