Difference between revisions of "Acetic acid"
(username removed) |
|||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A colorless, corrosive liquid with a strong [ | + | A colorless, corrosive liquid with a strong [[vinegar|vinegar]] smell. It is widely used in industry as a solvent and reagent. Pure acetic acid, (>99.7%) is called glacial acetic acid. Acetic acid is the active ingredient in vinegar in concentrations of about 5%, giving it an acidic flavor and a pungent odor. Acetic acid is found naturally in many fruits, plants, and wood. It is deleterious to [[metal|metals]] and may be harmful to [[oil%20paint|oil paintings]], [[watercolor%20paint|watercolors]], drawings and sketches. Acetic acid is used in the manufacture of [[cellulose%20acetate|acetates]] and [[plastic|plastics]], printing [[calico|calico]] and dyeing [[silk|silk]], in [[pesticide|pesticides]], photographic chemicals, pharmaceuticals, as an [[etching%20solution|etching agent]], [[bleaching%20agent|bleach]], and stain remover, and as a preservative in food processing. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 12:54, 6 January 2014
Description
A colorless, corrosive liquid with a strong Vinegar smell. It is widely used in industry as a solvent and reagent. Pure acetic acid, (>99.7%) is called glacial acetic acid. Acetic acid is the active ingredient in vinegar in concentrations of about 5%, giving it an acidic flavor and a pungent odor. Acetic acid is found naturally in many fruits, plants, and wood. It is deleterious to metals and may be harmful to oil paintings, watercolors, drawings and sketches. Acetic acid is used in the manufacture of acetates and plastics, printing Calico and dyeing Silk, in pesticides, photographic chemicals, pharmaceuticals, as an etching agent, bleach, and stain remover, and as a preservative in food processing.
Synonyms and Related Terms
glacial acetic acid; vinegar acid; ethanoic acid; ethylic acid; methanecarboxylic acid; Varigam toner; Fixer 6a; acide acétique (Fr.)
Other Properties
Miscible in water, ethanol, glycerol, ether, carbon tetrachloride. Insoluble in carbon disulfide.
pKa1 = 4.756
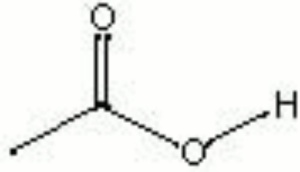
| Composition | CH3COOH |
|---|---|
| CAS | 64-19-7 |
| Melting Point | 16.7 |
| Density | 1.053 |
| Molecular Weight | mol. wt. = 60.05 |
| Refractive Index | 1.3718 |
| Boiling Point | 118 |
Hazards and Safety
Moderately combustible. Will corrode metals.
For glacial acetic acid: skin contact will produce burns; fumes can cause skin, eye and lung irritation; ingestion may be fatal.
Mallinckrodt Baker: MSDS
Comparisons
Authority
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p.7
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- John and Margaret Cannon, Dye Plants and Dyeing, Herbert Press, London, 1994
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: ref. index=1.3718
- Tom Rowland, Noel Riley, A-Z Guide to Cleaning, Conserving and Repairing Antiques, Constable and Co., Ltd., London, 1981
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.370