Difference between revisions of "Phthalocyanine dye"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
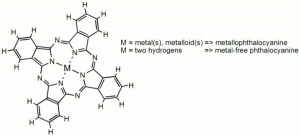
| − | A chromophore composed of a conjugated system of double bonds that forms a color complex with a metal. Phthalocyanine type choromophores occur naturally in chlorophyll and animal blood. They were first synthesized in the 1920s and commercially introduced in the 1930s. Because they are insoluble, phthlocyanine dyes are also classified as pigments. Examples include [ | + | A chromophore composed of a conjugated system of double bonds that forms a color complex with a metal. Phthalocyanine type choromophores occur naturally in chlorophyll and animal blood. They were first synthesized in the 1920s and commercially introduced in the 1930s. Because they are insoluble, phthlocyanine dyes are also classified as pigments. Examples include [[phthalocyanine%20green|phthalocyanine green]] and [[phthalocyanine%20blue|phthalocyanine blue]]. The dyes are not chemically reactive and must be physically attached to the substrate with a binder or adhesive. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 10:18, 10 May 2016
Description
A chromophore composed of a conjugated system of double bonds that forms a color complex with a metal. Phthalocyanine type choromophores occur naturally in chlorophyll and animal blood. They were first synthesized in the 1920s and commercially introduced in the 1930s. Because they are insoluble, phthlocyanine dyes are also classified as pigments. Examples include Phthalocyanine green and Phthalocyanine blue. The dyes are not chemically reactive and must be physically attached to the substrate with a binder or adhesive.
Synonyms and Related Terms
phthalocyanine dyes; phthlocyanine pigments; aza[18]annulene dye; Monastral blue
Other Properties
Insoluble in water
Sources Checked for Data in Record
- Website address 1 Comment: www.handprint.com
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Phthalocyanine (Accessed Nov. 9, 2005)