Difference between revisions of "Toluene"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | Colorless liquid solvent with a [ | + | Colorless liquid solvent with a [[benzene|benzene]] odor. Toluene is produced from the fractional distillation of coal tar. It is used as a solvent for paint, coatings, resins, as well as for most oils, rubber, polymers, and adhesives. Toluene is also used as a component in aviation fuel and for the manufacture of dyestuffs and explosives. Industrial grade toluene has been called toluol. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 12:52, 10 May 2016
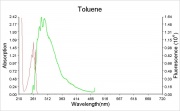
Description
Colorless liquid solvent with a Benzene odor. Toluene is produced from the fractional distillation of coal tar. It is used as a solvent for paint, coatings, resins, as well as for most oils, rubber, polymers, and adhesives. Toluene is also used as a component in aviation fuel and for the manufacture of dyestuffs and explosives. Industrial grade toluene has been called toluol.
Synonyms and Related Terms
toluol; methylbenzene; phenylmethane; methyl benzene
Other Properties
Soluble in ethanol, benzene, ether. Insoluble in water. Burns with a smoky flame.
| Composition | C6H5CH3 |
|---|---|
| CAS | 108-88-3 |
| Melting Point | -94.7 |
| Density | 0.866 |
| Molecular Weight | mol. wt.=92.13 |
| Refractive Index | 1.4967 |
| Boiling Point | 110.7 |
Hazards and Safety
Flammable. Flash point = 4C.
Toxic by ingestion, inhalation and skin absorption.
LINK: International Chemical Safety Card
Comparisons
Sources Checked for Data in Record
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9667; ref. index=1.4967
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.494