Difference between revisions of "Madder (Rubia peregrina) LC"
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File: | + | [[File:Madder.jpg|thumb|Indian madder / Photo by X. Zhang/]] |
== Description == | == Description == | ||
| Line 7: | Line 7: | ||
== Historical importance == | == Historical importance == | ||
| − | Rubia peregrina is called "wild madder". | + | ''Rubia peregrina'' is called "wild madder". |
| − | = Summary of results = | + | == Summary of results == |
| − | R. peregrina contains a peak at about 15 min that appears to be lucidin primeveroside (15, MW =564 Da), as well as an isomer | + | ''R. peregrina'' contains a peak at about 15 min that appears to be lucidin primeveroside (15, MW =564 Da), as well as an isomer |
| − | of this compound, whose structure we have not been able to determine. Confirming the assignment of the 564-Da peak as 15 is the presence of a peak with a mass (MW= 270 Da) and an elution time consistent with lucidin (16) in the HCl treated extract (Fig. c). Note also that Fig. a shows a number of small, unlabeled peaks. These are anthraquinones, judging from their UV/Visible spectra, but vary in size from sample to sample, even within the same | + | of this compound, whose structure we have not been able to determine. Confirming the assignment of the 564-Da peak as 15 is the presence of a peak with a mass (MW= 270 Da) and an elution time consistent with lucidin (16) in the HCl treated extract (Fig. c). Note also that Fig. a shows a number of small, unlabeled peaks. These are anthraquinones, judging from their UV/Visible spectra, but vary in size from sample to sample, even within the same species, and therefore are unreliable as markers [1]. |
| − | species, and therefore are unreliable as markers [1]. | ||
| − | = Analytical instrumentation and procedures = | + | == Analytical instrumentation and procedures == |
'''Extraction of plant material''' | '''Extraction of plant material''' | ||
| Line 33: | Line 32: | ||
Extracts of plant material or of dyed silk or wool were analyzed by HPLC with photodiode array and mass spectrometric detection using an Agilent 1100 high performance liquid chromatography system consisting of an automatic injector, a gradient pump, a Hewlett-Packard series 1100 photodiode array detector, and an Agilent series 1100 VL on-line atmospheric pressure ionization electrospray ionization mass spectrometer. Operation of the system and data analysis were done using ChemStation software, and detection was generally done in the negative ion [M-H]– mode. Separation of dye components was made, in the majority of cases, on a Vydac C18 reversed phase column (2.1 µm dia.×250 µm long; 5-µm particle size). Columns were eluted with acetonitrile-water gradients containing 0.1 % formic acid in both solvents. | Extracts of plant material or of dyed silk or wool were analyzed by HPLC with photodiode array and mass spectrometric detection using an Agilent 1100 high performance liquid chromatography system consisting of an automatic injector, a gradient pump, a Hewlett-Packard series 1100 photodiode array detector, and an Agilent series 1100 VL on-line atmospheric pressure ionization electrospray ionization mass spectrometer. Operation of the system and data analysis were done using ChemStation software, and detection was generally done in the negative ion [M-H]– mode. Separation of dye components was made, in the majority of cases, on a Vydac C18 reversed phase column (2.1 µm dia.×250 µm long; 5-µm particle size). Columns were eluted with acetonitrile-water gradients containing 0.1 % formic acid in both solvents. | ||
| − | = Chromatograms = | + | == Chromatograms == |
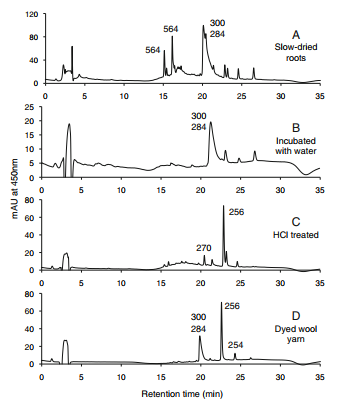
HPLC profiles for Rubia peregrina. Elution was monitored at 450 nm. The numbers over the peaks are the molecule masses of the principal of otherwise significant components [1]. | HPLC profiles for Rubia peregrina. Elution was monitored at 450 nm. The numbers over the peaks are the molecule masses of the principal of otherwise significant components [1]. | ||
| Line 40: | Line 39: | ||
| − | Sample information | + | == Sample information == |
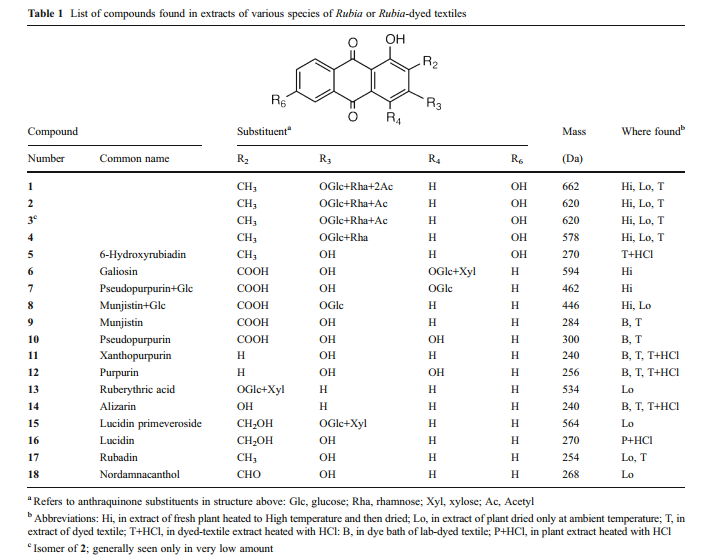
[[File:Madder table 1.PNG|center|frame|compounds found in extracts of various species of Rubia or Rubia-dyes textiles. By R. A. Laursen, Boston University ]] | [[File:Madder table 1.PNG|center|frame|compounds found in extracts of various species of Rubia or Rubia-dyes textiles. By R. A. Laursen, Boston University ]] | ||
| − | = Identified compounds = | + | == Identified compounds == |
[[[SliderGallery rightalign|~HPLC-DAD|.JPG~ UV-Vis|.jpg~ UV-Vis]]] | [[[SliderGallery rightalign|~HPLC-DAD|.JPG~ UV-Vis|.jpg~ UV-Vis]]] | ||
| Line 56: | Line 55: | ||
| − | [[Category:Analysis | + | [[Category:Dye Analysis]] |
| + | [[Category:Reference Materials]] | ||
| + | [[Category:Natural Dyes]] | ||
Latest revision as of 10:46, 26 July 2017
Description
Historical importance
Rubia peregrina is called "wild madder".
Summary of results
R. peregrina contains a peak at about 15 min that appears to be lucidin primeveroside (15, MW =564 Da), as well as an isomer of this compound, whose structure we have not been able to determine. Confirming the assignment of the 564-Da peak as 15 is the presence of a peak with a mass (MW= 270 Da) and an elution time consistent with lucidin (16) in the HCl treated extract (Fig. c). Note also that Fig. a shows a number of small, unlabeled peaks. These are anthraquinones, judging from their UV/Visible spectra, but vary in size from sample to sample, even within the same species, and therefore are unreliable as markers [1].
Analytical instrumentation and procedures
Extraction of plant material
Samples of plant specimens were extracted by heating about 10 mg of the ground or chopped, dry plant with 1.0 mL of methanol/water (1:1) at 65 °C for 1 h. The supernatant liquid was removed by pipet and was centrifuged at 12,000 rpm for about 5 min, after which the clear supernatant was subjected to analysis. This solution was diluted with methanol/water (1:1) if necessary.
Extraction of dyed textiles or fibers
Dyed textile or yarn specimens were extracted using a “soft” procedure, namely, by heating approximately 0.1–1 mg of fibers in 200 μL of a solution of pyridine/water/1.0 M oxalic acid in water (95:95:10) at 100 °C for 15 min.
HCl treated
The plant extracts were heated in 2 M HCl at 95 °C for 60 min to hydrolyze O-glycosides or to promote decarboxylation.
Analysis of dye components
Extracts of plant material or of dyed silk or wool were analyzed by HPLC with photodiode array and mass spectrometric detection using an Agilent 1100 high performance liquid chromatography system consisting of an automatic injector, a gradient pump, a Hewlett-Packard series 1100 photodiode array detector, and an Agilent series 1100 VL on-line atmospheric pressure ionization electrospray ionization mass spectrometer. Operation of the system and data analysis were done using ChemStation software, and detection was generally done in the negative ion [M-H]– mode. Separation of dye components was made, in the majority of cases, on a Vydac C18 reversed phase column (2.1 µm dia.×250 µm long; 5-µm particle size). Columns were eluted with acetonitrile-water gradients containing 0.1 % formic acid in both solvents.
Chromatograms
HPLC profiles for Rubia peregrina. Elution was monitored at 450 nm. The numbers over the peaks are the molecule masses of the principal of otherwise significant components [1].
Sample information
Identified compounds
References
[1] Chika Mouri and Richard Laursen "Identification of anthraquinone markers for distinguishing Rubia species in madder-dyed textiles by HPLC" Microchimica Acta October 2012, Volume 179, Issue 1–2, pp 105–113