Difference between revisions of "Young fustic (Cotinus coggygria ) LC"
| Line 14: | Line 14: | ||
== Analytical instrumentation and procedures == | == Analytical instrumentation and procedures == | ||
| + | HPLC-DAD-MS analysis was performed with an Agilent 1100 liquid chromatography system consisting of an automatic injector, a gradient pump, a HP series 1100 DAD, and an Agilent series 1100 VL on-line atmospheric pressure ionization electrospray ionization mass spectrometer. Separations were done on a Vydac 214TP52 analytical column (2.1 mm diameterX250 mm; 5-ím particle size). The column was eluted at a flow rate of 0.2 mL/min with a tertiary gradient of water (A),acetonitrile (B), and 1% (v/v) aqueous formic acid (C) with the following elution program: 0 min, 90% A, 5% B, 5% C; 0-55 min, a linear gradient to 35% A, 60% B, 5% C; 55-60 min, a linear gradient elution to 15% A, 80% B, 5% C; 60-62 min, isocratic elution at 15% A, 80% B, 5% C; 62-70 min gradient elution to 90% A, 5% B, 5% C; and reequilibration with the latter solvent for 15 min. The mass spectrometer was run both in the negative and positive ion mode. | ||
== Chromatograms == | == Chromatograms == | ||
Latest revision as of 09:24, 29 September 2017
Description
A natural yellow dyestuff obtained from the wood of the smoketree, Cotinus coggygria (formerly Rhus cotinus). This small tree, also called Venetian sumac, is native to southern Europe, the Middle East, India and China.
Historical importance
Summary of results
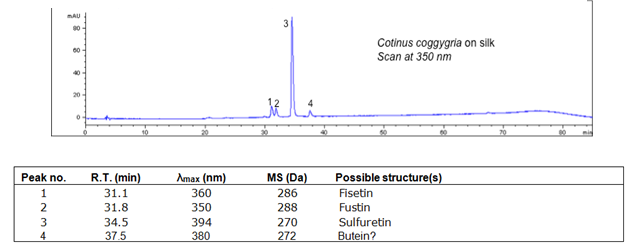
The primary coloring compounds are fisetin and sulferitin. Young fustic has poor lightfastness.
Analytical instrumentation and procedures
HPLC-DAD-MS analysis was performed with an Agilent 1100 liquid chromatography system consisting of an automatic injector, a gradient pump, a HP series 1100 DAD, and an Agilent series 1100 VL on-line atmospheric pressure ionization electrospray ionization mass spectrometer. Separations were done on a Vydac 214TP52 analytical column (2.1 mm diameterX250 mm; 5-ím particle size). The column was eluted at a flow rate of 0.2 mL/min with a tertiary gradient of water (A),acetonitrile (B), and 1% (v/v) aqueous formic acid (C) with the following elution program: 0 min, 90% A, 5% B, 5% C; 0-55 min, a linear gradient to 35% A, 60% B, 5% C; 55-60 min, a linear gradient elution to 15% A, 80% B, 5% C; 60-62 min, isocratic elution at 15% A, 80% B, 5% C; 62-70 min gradient elution to 90% A, 5% B, 5% C; and reequilibration with the latter solvent for 15 min. The mass spectrometer was run both in the negative and positive ion mode.
Chromatograms
Sample information
Identified compounds
| Compound | RT (min.) | MW | UV/vis | Other |
|---|---|---|---|---|
| Fisetin | 31.1 | 286 | 360 | Comments here |
| Fustin | 31.8 | 288 | 350 | |
| Sulfuretin | 34.5 | 270 | 394 | |
| Butein | 37.5 | 272 | 380 |
References
[1] [2] [3]