Difference between revisions of "Supercritical fluid"
Jump to navigation
Jump to search
| (7 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
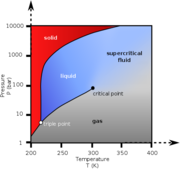
| − | [[File: | + | [[File:Carbon_dioxide_pressure-temperature_phase.png|thumb|Phase diagram for carbon dioxide]] |
== Description == | == Description == | ||
| Line 8: | Line 8: | ||
SCF | SCF | ||
| − | [[ | + | == Physical and Chemical Properties == |
| + | |||
| + | {|class="wikitable" style="text-align:center" | ||
| + | |+Table 1. Critical properties of various solvents (Reid et al., 1987) | ||
| + | |- | ||
| + | ! rowspan="2" | Solvent !! Molecular mass !! Critical temperature !! Critical pressure !! Critical density | ||
| + | |- | ||
| + | ! g/mol !! K !! Pascal (atm) !! g/cm<sup>3</sup> | ||
| + | |- | ||
| + | | [[Carbon dioxide]] (CO<sub>2</sub>) | ||
| + | | 44.01 || 304.1 || 7.38 (72.8) || 0.469 | ||
| + | |- | ||
| + | | [[water|Water]] (H<sub>2</sub>O)<sup>†</sup> | ||
| + | | 18.015 || 647.096 || 22.064 (217.755) || 0.322 | ||
| + | |- | ||
| + | | [[Methane]] (CH<sub>4</sub>) | ||
| + | | 16.04 || 190.4 || 4.60 (45.4) || 0.162 | ||
| + | |- | ||
| + | | Ethane (C<sub>2</sub>H<sub>6</sub>) | ||
| + | | 30.07 || 305.3 || 4.87 (48.1) || 0.203 | ||
| + | |- | ||
| + | | [[Propane]] (C<sub>3</sub>H<sub>8</sub>) | ||
| + | | 44.09 || 369.8 || 4.25 (41.9) || 0.217 | ||
| + | |- | ||
| + | | [[Ethylene]] (C<sub>2</sub>H<sub>4</sub>) | ||
| + | | 28.05 || 282.4 || 5.04 (49.7) || 0.215 | ||
| + | |- | ||
| + | | [[Propylene]] (C<sub>3</sub>H<sub>6</sub>) | ||
| + | | 42.08 || 364.9 || 4.60 (45.4) || 0.232 | ||
| + | |- | ||
| + | | [[Methanol]] (CH<sub>3</sub>OH) | ||
| + | | 32.04 || 512.6 || 8.09 (79.8) || 0.272 | ||
| + | |- | ||
| + | | [[Ethanol]] (C<sub>2</sub>H<sub>5</sub>OH) | ||
| + | | 46.07 || 513.9 || 6.14 (60.6) || 0.276 | ||
| + | |- | ||
| + | | [[Acetone]] (C<sub>3</sub>H<sub>6</sub>O) | ||
| + | | 58.08 || 508.1 || 4.70 (46.4) || 0.278 | ||
| + | |- | ||
| + | | [[Nitrous oxide]] (N<sub>2</sub>O) | ||
| + | | 44.013 || 306.57 || 7.35 (72.5) || 0.452 | ||
| + | |} | ||
| + | |||
== Resources and Citations == | == Resources and Citations == | ||
* Sung Mo Kang, Achim Unger, J.J. Morrell, 'The Effect of Supercritical Carbon Dioxide Extraction of Color Retention and Pesticide Reduction of Wooden Artifacts' ''JAIC'' 43(2) 151-160, 2004. | * Sung Mo Kang, Achim Unger, J.J. Morrell, 'The Effect of Supercritical Carbon Dioxide Extraction of Color Retention and Pesticide Reduction of Wooden Artifacts' ''JAIC'' 43(2) 151-160, 2004. | ||
| − | * Wikipedia: http://en.wikipedia.org/wiki/Supercritical_fluid ( | + | * Wikipedia: http://en.wikipedia.org/wiki/Supercritical_fluid (June 2020) |
| + | |||
| + | * Robert C. Reid ''The Properties of Gases and Liquids'', McGraw-Hill, 1987. | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 09:52, 14 June 2020
Description
A material compressed and heated to a point above its thermodynamic critical point. Supercritical fluids (SCF) have the unique ability to penetrate materials like a gas while also dissolving materials like a liquid. Carbon dioxide and water are the most commonly used supercritical fluids. A temperatures and pressures above the thermodynamic critical point, a material's liquid phase and the gas phase will have equal densities and are indistinguishable.
Synonyms and Related Terms
SCF
Physical and Chemical Properties
| Solvent | Molecular mass | Critical temperature | Critical pressure | Critical density |
|---|---|---|---|---|
| g/mol | K | Pascal (atm) | g/cm3 | |
| Carbon dioxide (CO2) | 44.01 | 304.1 | 7.38 (72.8) | 0.469 |
| Water (H2O)† | 18.015 | 647.096 | 22.064 (217.755) | 0.322 |
| Methane (CH4) | 16.04 | 190.4 | 4.60 (45.4) | 0.162 |
| Ethane (C2H6) | 30.07 | 305.3 | 4.87 (48.1) | 0.203 |
| Propane (C3H8) | 44.09 | 369.8 | 4.25 (41.9) | 0.217 |
| Ethylene (C2H4) | 28.05 | 282.4 | 5.04 (49.7) | 0.215 |
| Propylene (C3H6) | 42.08 | 364.9 | 4.60 (45.4) | 0.232 |
| Methanol (CH3OH) | 32.04 | 512.6 | 8.09 (79.8) | 0.272 |
| Ethanol (C2H5OH) | 46.07 | 513.9 | 6.14 (60.6) | 0.276 |
| Acetone (C3H6O) | 58.08 | 508.1 | 4.70 (46.4) | 0.278 |
| Nitrous oxide (N2O) | 44.013 | 306.57 | 7.35 (72.5) | 0.452 |
Resources and Citations
- Sung Mo Kang, Achim Unger, J.J. Morrell, 'The Effect of Supercritical Carbon Dioxide Extraction of Color Retention and Pesticide Reduction of Wooden Artifacts' JAIC 43(2) 151-160, 2004.
- Wikipedia: http://en.wikipedia.org/wiki/Supercritical_fluid (June 2020)
- Robert C. Reid The Properties of Gases and Liquids, McGraw-Hill, 1987.