Difference between revisions of "Polyvinyl acetate"
| (8 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:1983.416-SC3200.jpg|thumb|]] | + | [[File:1983.416-SC3200.jpg|thumb|Diamond Totem<br>MFA# 1983.416]] |
| + | [[File:PVAC lamp 20094954.jpg|thumb|PVAC bubble lamp<br> MFA# 2009.4954]] | ||
== Description == | == Description == | ||
| − | A | + | A white, nontoxic thermoplastic resin prepared by the polymerization of vinyl acetate<ref>https://www.britannica.com/science/polyvinyl-acetate</ref>. Polyvinyl acetate (PVAC) was discovered in 1912 by Dr. Fritz Klatte in Germany. It is one of the most widely used water-dispersed resins. Polyvinyl acetate water-based emulsions is often applied as a liquid that hardens to a solid when the water evaporates. PVAC resins produce clear, hard films that have good weather resistance and withstand water, grease, oil, and petroleum fuels. Additional properties are high initial tack, almost invisible bond line, softening at 30-45C, good biodegradation resistance, poor resistance to creep under load, and low cost. |
[[File:pva.jpg|thumb|Polyvinyl acetate solution]] | [[File:pva.jpg|thumb|Polyvinyl acetate solution]] | ||
| + | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
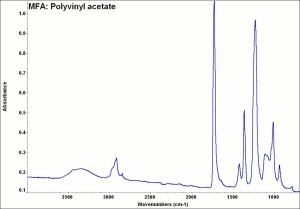
[[[SliderGallery rightalign|MFA- Polyvinyl acetate.jpg~FTIR]]] | [[[SliderGallery rightalign|MFA- Polyvinyl acetate.jpg~FTIR]]] | ||
| Line 12: | Line 14: | ||
== Applications == | == Applications == | ||
| − | * Latex house paints | + | * Latex house paints |
| + | * Artists' media (since 1945) | ||
* Common household water-based white glues | * Common household water-based white glues | ||
* Hot-melt adhesives, sealants, fabric finishing, plastic wood, and inks. | * Hot-melt adhesives, sealants, fabric finishing, plastic wood, and inks. | ||
== Personal Risks == | == Personal Risks == | ||
| − | The polymer is | + | The polymer is generally safe for handling. The pH of aqueous emulsions is usually acidic. The vinyl acetate monomer is flammable and reactive and is on the Special Health Hazardous Substance List<ref>https://www.nj.gov/health/eoh/rtkweb/documents/fs/1998.pdf</ref>. |
Fisher Scientific: [https://fscimage.fishersci.com/msds/97152.htm MSDS] | Fisher Scientific: [https://fscimage.fishersci.com/msds/97152.htm MSDS] | ||
| Line 23: | Line 26: | ||
== Collection Risks == | == Collection Risks == | ||
| − | May release | + | May release [[acetic%20acid|acetic acid]] on curing, aging and deterioration. Susceptible to hydrolysis which can increase the amount of acid released as well as interfere with the adhesive function of the material. The amounts of acid released are variable and depend on the formulation. |
| − | + | ||
| + | ==Environmental Risks== | ||
| + | The monomer is flammable but the polymer is generally considered stable. | ||
| + | |||
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| Line 36: | Line 42: | ||
* Density = 1.17-1.20 | * Density = 1.17-1.20 | ||
* Refractive Index = 1.46-1.47 | * Refractive Index = 1.46-1.47 | ||
| + | |||
| + | ==Working Properties== | ||
== Comparisons == | == Comparisons == | ||
| Line 44: | Line 52: | ||
== Resources and Citations == | == Resources and Citations == | ||
| + | <references/> | ||
* Contributions: Catherine Stephens, AIC Plastics Panel, 2020. | * Contributions: Catherine Stephens, AIC Plastics Panel, 2020. | ||
| Line 72: | Line 81: | ||
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | * | + | * History of Plastics: www.nswpmith.com.au/historyofplastics.html .. vinyl acetate was discovered in Germany by Dr. Fritz Klatte in 1912 with patents for its preparation |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 08:00, 13 August 2020
Description
A white, nontoxic thermoplastic resin prepared by the polymerization of vinyl acetate[1]. Polyvinyl acetate (PVAC) was discovered in 1912 by Dr. Fritz Klatte in Germany. It is one of the most widely used water-dispersed resins. Polyvinyl acetate water-based emulsions is often applied as a liquid that hardens to a solid when the water evaporates. PVAC resins produce clear, hard films that have good weather resistance and withstand water, grease, oil, and petroleum fuels. Additional properties are high initial tack, almost invisible bond line, softening at 30-45C, good biodegradation resistance, poor resistance to creep under load, and low cost.
Synonyms and Related Terms
PVAC; PVAc; PVA (incorrect but commonly used); poly(vinyl acetate); polyvinylacetate; poli(acetato de vinilo) (Esp.); acétate de polyvinyle (Fr.); polivinil acetato (It.); poliacetato de vinilo (Port.); vinyl emulsion; vinyl acetate plastic
Examples: Vinamul [Vinyl Products]; AYAT [Union Carbide]; Elmers® Glue-All [Borden]; Duratite White Glue [DAP]; Gelva® [Solutia]; Rivit Glue; Resin W; Polymer Tempera [Borden]; Vinavyl; Everflex BG [W.R.Grace & Co.]; Polyco [DAP]; Vinnapas [Wacker]; Mowilith [Hoechst]; polyvinylacetat (Deut.)
Applications
- Latex house paints
- Artists' media (since 1945)
- Common household water-based white glues
- Hot-melt adhesives, sealants, fabric finishing, plastic wood, and inks.
Personal Risks
The polymer is generally safe for handling. The pH of aqueous emulsions is usually acidic. The vinyl acetate monomer is flammable and reactive and is on the Special Health Hazardous Substance List[2].
Fisher Scientific: MSDS
Collection Risks
May release Acetic acid on curing, aging and deterioration. Susceptible to hydrolysis which can increase the amount of acid released as well as interfere with the adhesive function of the material. The amounts of acid released are variable and depend on the formulation.
Environmental Risks
The monomer is flammable but the polymer is generally considered stable.
Physical and Chemical Properties
- Soluble in benzene, chloroform, methanol, acetone, butyl acetate.
- Insoluble in ligroin, diethyl ether, butanol, turpentine, water, oils.
- Can absorb 3-5% water when immersed for 16 hours at 60 C.
- Burns with a dark yellow flame that smells of acetic acid.
- Composition : [-CH2CH(OOCCH3)-]n
- CAS : 9003-20-7
- Melting Point (Tg) = 30-45
- Density = 1.17-1.20
- Refractive Index = 1.46-1.47
Working Properties
Comparisons
General Characteristics of Polymers
Physical Properties for Selected Thermoplastic Resins
Resources and Citations
- Contributions: Catherine Stephens, AIC Plastics Panel, 2020.
- A.E.Corey, P.M.Draghetti, J.Fantl, "Polyvinyl Acetate Emulsions and Polyvinyl Alcohol for Adhesives" in Handbook of Adhesives, I.Skeist (ed.), Van Nostrand Reinhold, New York, 1977, p.465-483.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 849
- Wikipedia: http://en.wikipedia.org/wiki/Polyvinyl_acetate (Accessed Jan.6 2006)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Jo Crook, Tom Learner, Modern Paints, Watson-Guptill Publications, New York, 2000 Comment: First paint marketed in 1945 - Polymer Tempera by Borden
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- History of Plastics: www.nswpmith.com.au/historyofplastics.html .. vinyl acetate was discovered in Germany by Dr. Fritz Klatte in 1912 with patents for its preparation