Difference between revisions of "Benzoic acid"
Jump to navigation
Jump to search
| Line 8: | Line 8: | ||
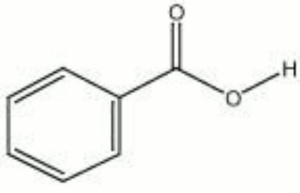
[[[SliderGallery rightalign|benzoic acid.jpg~Chemical structure]]] | [[[SliderGallery rightalign|benzoic acid.jpg~Chemical structure]]] | ||
| + | |||
| + | == Risks == | ||
| + | |||
| + | Mildly irritating to skin and membranes. Moderately toxic by ingestion. Combustible. | ||
| + | |||
| + | Fisher Scientific: [https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-b/S25195.pdf SDS] | ||
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| Line 33: | Line 39: | ||
| 249.2 | | 249.2 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Resources and Citations == | == Resources and Citations == | ||
Revision as of 16:23, 14 December 2020
Description
A white, crystalline powder obtained from Benzoin resin or other balsams. Benzoic acid is used as a mordant in calico printing, as an antifungal agent and for organic synthetic.
Synonyms and Related Terms
acid of benzoin; carboxybenzene; benzenecarboxylic acid; phenyl carboxylic acid; phenylformic acid; dracylic acid
Risks
Mildly irritating to skin and membranes. Moderately toxic by ingestion. Combustible.
Fisher Scientific: SDS
Physical and Chemical Properties
Soluble in ethanol, ether, chloroform, benzene, carbon disulfide, carbon tetrachloride, turpentine. Slightly soluble in water.
| Composition | C6H5COOH |
|---|---|
| CAS | 65-85-0 |
| Melting Point | 122.4 |
| Density | 1.2659 |
| Molecular Weight | mol. wt.=122.1 |
| Boiling Point | 249.2 |
Resources and Citations
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1122
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 777
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998