Difference between revisions of "Arsenic pentoxide"
Jump to navigation
Jump to search
| Line 8: | Line 8: | ||
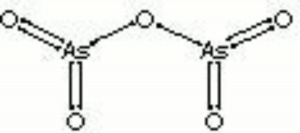
[[[SliderGallery rightalign|arsenic pentoxide.jpg~Chemical structure]]] | [[[SliderGallery rightalign|arsenic pentoxide.jpg~Chemical structure]]] | ||
| + | |||
| + | == Risks == | ||
| + | |||
| + | Highly toxic by ingestion and inhalation. Potential carcinogen and mutagen. | ||
| + | |||
| + | Fisher Scientific: [https://fscimage.fishersci.com/msds/02088.htm MSDS] | ||
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| Line 30: | Line 36: | ||
| mol. wt. = 229.84 | | mol. wt. = 229.84 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Resources and Citations == | == Resources and Citations == | ||
Latest revision as of 12:47, 15 December 2020
Description
White deliquescent crystals that are highly toxic. Arsenic pentoxide is used in insecticides, fungicides, textile dyeing and printing, and in glass manufacturing.
Synonyms and Related Terms
arsenic (V) oxide; arsenic anhydride; arsenic acid anhydride
Risks
Highly toxic by ingestion and inhalation. Potential carcinogen and mutagen.
Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in water, ethanol.
| Composition | As2O5 |
|---|---|
| CAS | 1303-28-2 |
| Melting Point | 315 |
| Density | 4.086 |
| Molecular Weight | mol. wt. = 229.84 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 839