Difference between revisions of "Ammonium bifluoride"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
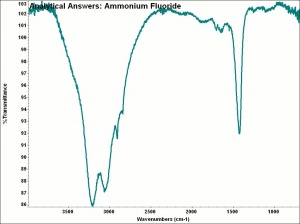
| − | + | [[[SliderGallery rightalign|aaiNH4F.jpg~FTIR|ammonium bifluoride.jpg~Chemical structure]]] | |
| − | White, deliquescent crystals that are corrosive to most materials. Ammonium bifluoride is used as an etchant for [ | + | White, deliquescent crystals that are corrosive to most materials. Ammonium bifluoride is used as an etchant for [[glass|glass]] and [[aluminum|aluminum]]. It is also used to sterilizing brewing and dairy equipment. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 7: | Line 7: | ||
ammonium acid fluoride; ammonium hydrogen fluoride; white acid | ammonium acid fluoride; ammonium hydrogen fluoride; white acid | ||
| − | [ | + | == Risks == |
| + | * Corrosive to skin, eyes and lungs. Toxic by ingestion. | ||
| + | * IntegraChem: [http://www.integrachem.com/msds/A599_25096_101.pdf SDS] | ||
| − | == | + | == Physical and Chemical Properties == |
Soluble in water, ethanol. pH=3.5 | Soluble in water, ethanol. pH=3.5 | ||
| Line 22: | Line 24: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 124.6 | + | | 124.6 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.211 | + | | 1.211 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 33: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 240 | + | | 240 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 49: | Line 43: | ||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| + | |||
| + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 299 | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 523 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 523 | ||
Latest revision as of 12:36, 26 April 2022
Description
White, deliquescent crystals that are corrosive to most materials. Ammonium bifluoride is used as an etchant for Glass and Aluminum. It is also used to sterilizing brewing and dairy equipment.
Synonyms and Related Terms
ammonium acid fluoride; ammonium hydrogen fluoride; white acid
Risks
- Corrosive to skin, eyes and lungs. Toxic by ingestion.
- IntegraChem: SDS
Physical and Chemical Properties
Soluble in water, ethanol. pH=3.5
| Composition | NH4HF2 |
|---|---|
| CAS | 1341-49-7 |
| Melting Point | 124.6 C |
| Density | 1.211 g/ml |
| Molecular Weight | mol. wt. = 57.04 |
| Boiling Point | 240 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 299
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 523