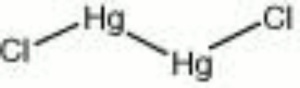Difference between revisions of "Calomel"
Jump to navigation
Jump to search
(username removed) |
|||
| (5 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A brownish-white ore containing [ | + | A brownish-white ore containing [[mercurous chloride]]. The name 'Calomel' is also used to refer to pure mercurous chloride. It has been used as a [[fungicide]], [[insecticide]], and topical anesthetic. One Peruvian cabinet decorated with mopa-mopa, was found to contain calomel as a white pigment (Newman 2015). |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | mercurous chloride; mercury subchloride; mercury monochloride; mercury protochloride; | + | mercury chloride; mercurous chloride; mercury subchloride; mercury monochloride; mercury protochloride; precipité blanc; Calogreen; Cyclosan, M-C Turf fungicide; calomel (Fr.); calomelano (Esp., Port.); Calomel (Deut.) |
[[[SliderGallery rightalign|calomel.jpg~Chemical structure]]] | [[[SliderGallery rightalign|calomel.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| − | Tabular crystals or mass. Cleavage = good in one direction. Luster = adamantine | + | * Toxic by ingestion, inhalation and skin absorption. |
| + | * echemi: [https://www.echemi.com/sds/calomel-pid_Rock20677.html SDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
| + | |||
| + | * Tabular crystals or mass. | ||
| + | * Cleavage = good in one direction. | ||
| + | * Luster = adamantine | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 25: | Line 32: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 7.15 | + | | 7.15 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 41: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | + | * R. Newman, E. Kaplan, M. Derrick, “Mopa mopa: Scientific analysis and history of an unusual South American resin used by the Inka and artisans in Pasto, Colombia,” Journal of the American Institute for Conservation 54 (2015): 123-148. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5957 |
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 200 |
| − | * | + | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 |
| − | * | + | * ''Encyclopedia Britannica'', http://www.britannica.com |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 15:11, 18 May 2022
Description
A brownish-white ore containing Mercurous chloride. The name 'Calomel' is also used to refer to pure mercurous chloride. It has been used as a Fungicide, Insecticide, and topical anesthetic. One Peruvian cabinet decorated with mopa-mopa, was found to contain calomel as a white pigment (Newman 2015).
Synonyms and Related Terms
mercury chloride; mercurous chloride; mercury subchloride; mercury monochloride; mercury protochloride; precipité blanc; Calogreen; Cyclosan, M-C Turf fungicide; calomel (Fr.); calomelano (Esp., Port.); Calomel (Deut.)
Risks
- Toxic by ingestion, inhalation and skin absorption.
- echemi: SDS
Physical and Chemical Properties
- Tabular crystals or mass.
- Cleavage = good in one direction.
- Luster = adamantine
| Composition | Hg2Cl2 |
|---|---|
| CAS | 10112-91-1 |
| Mohs Hardness | 1.5 |
| Density | 7.15 g/ml |
| Molecular Weight | mol. wt. = 472.09 |
| Refractive Index | 1.9-2.0; 2.6-2.7 |
Resources and Citations
- R. Newman, E. Kaplan, M. Derrick, “Mopa mopa: Scientific analysis and history of an unusual South American resin used by the Inka and artisans in Pasto, Colombia,” Journal of the American Institute for Conservation 54 (2015): 123-148.
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5957
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 200
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Encyclopedia Britannica, http://www.britannica.com
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993