Difference between revisions of "Ceresin wax"
Jump to navigation
Jump to search
(username removed) |
|||
| (4 intermediate revisions by 3 users not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A white or slightly yellowish [ | + | A white or slightly yellowish [[mineral wax]] prepared from purified and decolorized [[ozocerite]]. Ceresin is refined by treating powdered ozocerite with concentrated [[sulfuric acid]] then filtering through animal [[charcoal]]. The resultant wax is similar to [[paraffin wax|paraffin]], but is harder and has a higher melting point. Ceresin is composed of a wide range of long chain saturated hydrocarbons ranging from C20 to C32. Ceresin is used for candles, textile and paper sizing, floor polish, waterproofing, shoe polishes, and leather coating. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | + | cérésine (Fr.); ceresina (Esp., It.); purified ozocerite; earth wax; mineral wax; cerosin; ceresine | |
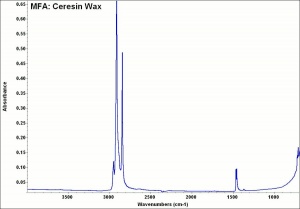
[[[SliderGallery rightalign|MFA- Ceresin Wax.jpg~FTIR]]] | [[[SliderGallery rightalign|MFA- Ceresin Wax.jpg~FTIR]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Combustible. | ||
| + | == Physical and Chemical Properties == | ||
Soluble in ethanol, benzene, chloroform, naphtha. Insoluble in water. Unaffected by acids or alkalis. | Soluble in ethanol, benzene, chloroform, naphtha. Insoluble in water. Unaffected by acids or alkalis. | ||
| Line 17: | Line 20: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 61-78 | + | | 61-78 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.91-.92 | + | | 0.91-.92 g/ml |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 31: | Line 30: | ||
[[media:download_file_31.pdf|Properties of Natural Waxes]] | [[media:download_file_31.pdf|Properties of Natural Waxes]] | ||
| − | + | == Resources and Citations == | |
| − | |||
| − | == | ||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 568 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 568 | ||
| Line 51: | Line 48: | ||
* ''A History of Technology'', Charles Singer, E.J. Holmyard, A.R. Hall (eds.), Clarendon Press, Oxford, Volume 1: From Early times to Fall of Ancient Empires, 1954 | * ''A History of Technology'', Charles Singer, E.J. Holmyard, A.R. Hall (eds.), Clarendon Press, Oxford, Volume 1: From Early times to Fall of Ancient Empires, 1954 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Ozokerite (Accessed Feb. 10, 2006) |
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
Latest revision as of 09:30, 24 May 2022
Description
A white or slightly yellowish Mineral wax prepared from purified and decolorized Ozocerite. Ceresin is refined by treating powdered ozocerite with concentrated Sulfuric acid then filtering through animal Charcoal. The resultant wax is similar to paraffin, but is harder and has a higher melting point. Ceresin is composed of a wide range of long chain saturated hydrocarbons ranging from C20 to C32. Ceresin is used for candles, textile and paper sizing, floor polish, waterproofing, shoe polishes, and leather coating.
Synonyms and Related Terms
cérésine (Fr.); ceresina (Esp., It.); purified ozocerite; earth wax; mineral wax; cerosin; ceresine
Risks
- Combustible.
Physical and Chemical Properties
Soluble in ethanol, benzene, chloroform, naphtha. Insoluble in water. Unaffected by acids or alkalis.
| Melting Point | 61-78 C |
|---|---|
| Density | 0.91-.92 g/ml |
Comparisons
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 568
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2033; mp=61-78 C
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: mp=65-80 C
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- E.J.LaBarre, Dictionary and Encyclopedia of Paper and Paper-making, Swets & Zeitlinger, Amsterdam, 1969
- John S. Mills, Raymond White, The Organic Chemistry of Museum Objects, Butterworth Heineman, London, 2nd ed., 1994
- A History of Technology, Charles Singer, E.J. Holmyard, A.R. Hall (eds.), Clarendon Press, Oxford, Volume 1: From Early times to Fall of Ancient Empires, 1954
- Wikipedia: http://en.wikipedia.org/wiki/Ozokerite (Accessed Feb. 10, 2006)
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000